Injection Guide Overview and Goals of This Guide ����������������������������������� 1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
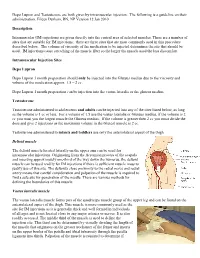
An Intramuscular Injection Is an Injection Given Directly Into The
Depo Lupron and Testosterone are both given by intramuscular injection. The following is a guideline on their administration. Eileen Durham, RN, NP Version 12 Jan 2010 Description Intramuscular (IM) injections are given directly into the central area of selected muscles. There are a number of sites that are suitable for IM injections; there are three sites that are most commonly used in this procedure described below. The volume of viscosity of the medication to be injected determines the site that should be used. IM injections cause stretching of the muscle fiber so the larger the muscle used the less discomfort. Intramuscular Injection Sites Depo Lupron Depo Lupron 3 month preparation should only be injected into the Gluteus medius due to the viscosity and volume of the medication approx. 1.5 – 2 cc. Depo Lupron 1 month preparation can be injection into the vastus lateralis or the gluteus medius. Testosterone Testosterone administered to adolescents and adults can be injected into any of the sites listed below, as long as the volume is 1 cc or less. For a volume of 1.5 use the vastus lateralis or Gluteus medius, if the volume is 2 cc you must you the largest muscle the Gluteus medius. If the volume is greater then 2 cc you must divide the dose and give 2 injections as the maximum volume in the Gluteal muscle is 2 cc. Testosterone administered to infants and toddlers use only the anteriolateral aspect of the thigh. Deltoid muscle The deltoid muscle located laterally on the upper arm can be used for intramuscular injections. -

Intravenous Therapy Procedure Manual
INTRAVENOUS THERAPY PROCEDURE MANUAL - 1 - LETTER OF ACCEPTANCE __________________________________________ hereby approves (Facility) the attached Reference Manual as of _____________________. (Date) The Intravenous Therapy Procedure Manual will be reviewed at least annually or more often when deemed appropriate. Revisions will be reviewed as they occur. Current copies of the Intravenous Therapy Procedure Manual shall be maintained at each appropriate nursing station. I have reviewed this manual and agree to its approval. __________________________ (Administrator) __________________________ (Director of Nursing) __________________________ (Medical Director) - 2 - TABLE OF CONTENTS TABLE OF CONTENTS INTRODUCTION A. Purpose 1 B. Local Standard of Practice 1 RESPONSIBILITIES A. Responsibilities: M Chest Pharmacy 1 B. Responsibilities: Administrator 1 C. Responsibilities: Director of Nursing Services (DON/DNS) 1 D. Skills Validation 2 AMENDMENTS GUIDELINES A. Resident Candidacy for IV Therapy 1 B. Excluded IV Medications and Therapies 1 C. Processing the IV Order 1 D. IV Solutions/Medications: Storage 2 E. IV Solutions/Medications: Handling 3 F. IV Solutions and Supplies: Destroying and Returning 4 G. IV Tubing 5 H. Peripheral IV Catheters and Needles 6 I. Central Venous Devices 7 J. Documentation and Monitoring 8 K. IV Medication Administration Times 9 L. Emergency IV Supplies 10 I TABLE OF CONTENTS PROTOCOLS A. IV Antibiotic 1 1. Purpose 2. Guidelines 3. Nursing Responsibilities B. IV Push 2 1. Purpose 2. Guidelines C. Anaphylaxis Allergic Reaction 4 1. Purpose 2. Guidelines 3. Nursing Responsibilities and Interventions 4. Signs and Symptoms of Anaphylaxis 5. Drugs Used to Treat Anaphylaxis 6. Physician Protocol PRACTICE GUIDELINES A. Purpose 1 B. Personnel 1 C. Competencies 1 D. -

Sodium Chloride Injection USP 0.9% Prefilled Syringes, for Intravascular Use Only ------WARNINGS and PRECAUTIONS ------Initial U.S
HIGHLIGHTS OF PRESCRIBING INFORMATION --------------------- DOSAGE FORMS AND STRENGTHS --------------------- These highlights do not include all the information needed to use • Supplied as a clear, colorless, odorless, sterile solution of Sodium Sodium Chloride Injection USP 0.9% safely and effectively. See Chloride 0.9% for intravenous administration (3) full prescribing information for Sodium Chloride Injection USP • Supplied in 50 mL and 125 mL prefilled syringes (3) 0.9%. ------------------------------- CONTRAINDICATIONS ------------------------------- • None (4) Sodium Chloride Injection USP 0.9% Prefilled Syringes, For Intravascular Use Only ----------------------- WARNINGS AND PRECAUTIONS ----------------------- Initial U.S. Approval: 2006 • Remove all air from the syringe and associated tubing prior to injection. (5.1) ----------------------------INDICATIONS AND USAGE ---------------------------- • For use in flushing compatible contrast agents through Liebel- • May cause fluid overload in patients with congestive heart failure, Flarsheim intravenous administration sets into indwelling severe renal insufficiency, and in clinical states with edema, sodium retention, or hypernatremia. (5.3) intravascular access devices (1) • Establish intravascular catheter patency prior to administration. ----------------------- DOSAGE AND ADMINISTRATION ----------------------- (5.4) • For single patient use only. (2) • Determine the volume of flush based on the imaging procedure, ------------------------------ ADVERSE REACTIONS ------------------------------ -

Injection Technique 1: Administering Drugs Via the Intramuscular Route
Copyright EMAP Publishing 2018 This article is not for distribution except for journal club use Clinical Practice Keywords Intramuscular injection/ Medicine administration/Absorption Practical procedures This article has been Injection technique double-blind peer reviewed Injection technique 1: administering drugs via the intramuscular route rugs administered by the intra- concerns that nurses are still performing Author Eileen Shepherd is clinical editor muscular (IM) route are depos- outdated and ritualistic practice relating to at Nursing Times. ited into vascular muscle site selection, aspirating back on the syringe Dtissue, which allows for rapid (Greenway, 2014) and skin cleansing. Abstract The intramuscular route allows absorption into the circulation (Dough- for rapid absorption of drugs into the erty and Lister, 2015; Ogston-Tuck, 2014). Site selection circulation. Using the correct injection Complications of poorly performed IM Four muscle sites are recommended for IM technique and selecting the correct site injection include: administration: will minimise the risk of complications. l Pain – strategies to reduce this are l Vastus lateris; outlined in Box 1; l Rectus femoris Citation Shepherd E (2018) Injection l Bleeding; l Deltoid; technique 1: administering drugs via l Abscess formation; l Ventrogluteal (Fig 1, Table 1). the intramuscular route. Nursing Times l Cellulitis; Traditionally the dorsogluteal (DG) [online]; 114: 8, 23-25. l Muscle fibrosis; muscle was used for IM injections but this l Injuries to nerves and blood vessels muscle is in close proximity to a major (Small, 2004); blood vessel and nerves, with sciatic nerve l Inadvertent intravenous (IV) access. injury a recognised complication (Small, These complications can be avoided if 2004). -

Administering a Subcutaneous Injection
Inject medication slowly and release skin Will the injection hurt? Children may describe a pinching/stinging or bee-sting sensation during Our Lady’s Children’s Leave needle in place for 5-10 seconds after and just after the injection. It is normal for the Hospital, Crumlin, Dublin injecting medication, if possible injection site to be a little red and tender. It is 12 Remove needle swiftly and smoothly expected that children may be afraid of injections. It is important to be honest and explain the Wipe area gently with cotton wool, do not rub injection in a manner that they can understand. ….where children’s health comes first as this may be sore. Apply a plaster if needed. How to reduce any discomfort: ADVICE FOR • Prepare your child, explain why this is necessary PARENTS/GUARDIANS/CHILDREN and how you will give the injection Step 4. Dispose of the Needle/Syringe • Use distraction and play to amuse your child Administering a • Encourage them to practice on their teddy or doll. For infants, give them a soother/comforter if Subcutaneous Injection Dispose of needle and syringe immediately into they use one a sharps bin • Ensure clothing over the injection site is not tight My child has a bleeding disorder: seek medical advice before giving injections to your child. Auto-injector: Some children use an automatic injection device, often called a ‘rocket’. Specific Instructions: ……………………………………………………………………… ………………………………………………………………………. Developed by: Naomi Bartley, Clinical Placement Coordinator. CONTACT DETAILS Example of an Auto-Injector st Date issued: July 2014, October 2012 : 1 edn. Ward / Dept. Name: _________________ Date of review: July 2017 Telephone: ___________________ Follow the manufacture’s instructions for ©2014, Our Lady’s Children’s Hospital Crumlin, Dublin 12. -

22428 Moxifloxacin Statisical PREA
U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research Office of Translational Science Office of Biostatistics S TATISTICAL R EVIEW AND E VALUATION CLINICAL STUDIES NDA/Serial Number: 22,428 Drug Name: Moxifloxacin AF (moxifloxacin hydrochloride ophthalmic solution) 0.5% Indication(s): Treatment of bacterial conjunctivitis Applicant: Alcon Pharmaceuticals, Ltd. Date(s): Letter date:21 May 2010; Filing date: 18 June, 2010; PDUFA goal date: 19 November 2010 Review Priority: Priority Biometrics Division: Anti-infective and Ophthalmology Products Statistical Reviewer: Mark. A. Gamalo, Ph.D. Concurring Reviewers: Yan Wang, Ph.D. Medical Division: Anti-infective and Ophthalmology Products Clinical Reviewer: Lucious Lim, M.D. Project Manager: Lori Gorski Keywords: superiority, Moxifloxacin hydrochloride ophthalmic solution, bacterial conjunctivitis, bulbar conjunctival injection, conjunctival discharge/exudate TABLE OF CONTENTS TABLE OF CONTENTS................................................................................................................ 2 LIST OF TABLES.......................................................................................................................... 3 1. EXECUTIVE SUMMARY ...................................................................................................... 4 1. 1 Conclusions and Recommendations..................................................................................... 4 1. 2 Brief Overview of Clinical Studies ..................................................................................... -

Self-Administering a Vitamin B12 Injection
Self-administering a Vitamin B12 injection This is usually given as an intramuscular injection, every 2-3 months. Alternatives to an intramuscular injection are: Oral Vitamin B12 at a dose of at least 1000mcg per day. • Available as tablets or a spray. They can be bought over the counter and available at most health food stores and pharmacies. • Oral Vitamin B12 is not recommended if: - If you have a bowel condition such as inflammatory bowel disease, Coeliac disease, small bowel overgrowth, bile acid malabsorption and short bowel (you will require injections) - You require an initial loading of B12 (soon after diagnosis) It is important to monitor your symptoms if you change to oral B12. If symptoms return, then the oral/sublingual dose can be increased to 2000mcg or you may need to consider starting back on injections. https://www.hollandandbarrett.com/shop/product/betteryou-pure-energy-b12-boost-oral-spray-60099160?skuid=099160 https://www.hollandandbarrett.com/search?query=%20Vitamin%20B12%20Tablets&utm_medium=cpc&utm_source=google&isSearch=true# gclid=EAIaIQobChMIh5nLgOH26AIVxLTtCh0JIA_GEAAYASAAEgJL-fD_BwE&gclsrc=aw.ds Subcutaneous (SC) injection; this is off-licence but still effective. It is considered an easier method of administration. It is how insulin and blood thinning medication are usually self-administered. Equipment needed to self-inject - Prescribed medicine - 1 syringe (2 ml) - 2 needles (1 for drawing up the drug and 1 for administration – you can use the same size needle for both). o For an IM injection; the needle gauge should be 19-25. The needle length is 1- 1 ½ inches (up to 3 inches for larger adults) o For a SC injection; the needle gauge should be 25-27. -

Fascia Iliaca Block in the Emergency Department for Hip Fracture
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Serveur académique lausannois Pasquier et al. BMC Geriatrics (2019) 19:180 https://doi.org/10.1186/s12877-019-1193-0 RESEARCH ARTICLE Open Access Fascia iliaca block in the emergency department for hip fracture: a randomized, controlled, double-blind trial Mathieu Pasquier1, Patrick Taffé2, Olivier Hugli1, Olivier Borens3, Kyle Robert Kirkham4 and Eric Albrecht5* Abstract Background: Hip fracture causes moderate to severe pain and while fascia iliaca block has been reported to provide analgesic benefit, most previous trials were unblinded, with subsequent high risks of performance, selection and detection biases. In this randomized, control double-blind trial, we tested the hypothesis that a fascia iliaca block provides effective analgesia for patients suffering from hip fracture. Methods: Thirty ASA I-III hip fracture patients over 70 years old, who received prehospital morphine, were randomized to receive either a fascia iliaca block using 30 ml of bupivacaine 0.5% with epinephrine 1:200,000 or a sham injection with normal saline. The fascia iliaca block was administered by emergency medicine physicians trained to perform an anatomic landmark-based technique. The primary outcome was the comparison between groups of the longitudinal pain score profiles at rest over the first 45 min following the procedure (numeric rating scale, 0–10). Secondary outcomes included the longitudinal pain score profiles on movement and the comparison over 4 h, 8 h, 12 h, and 24 h after the procedure, along with cumulative intravenous morphine consumption at 24 h. Results: At baseline, the fascia iliaca group had a lower mean pain score than the sham injection group, both at rest (difference = − 0.9, 95%CI [− 2.4, 0.5]) and on movement (difference = − 0.9, 95%CI [− 2.7; 0.9]). -

Informed Consent for Medication, Zyprexa
DEPARTMENT OF HEALTH SERVICES STATE OF WISCONSIN Division of Care and Treatment Services 42 CFR483.420(a)(2) F-24277 (09/2016) DHS 134.31(3)(o) DHS 94.03 & 94.09 §§ 51.61(1)(g) & (h) INFORMED CONSENT FOR MEDICATION Dosage and / or Side Effect information last revised on 04/16/2021 Completion of this form is voluntary. If not completed, the medication cannot be administered without a court order unless in an emergency. This consent is maintained in the client’s record and is accessible to authorized users. Name – Patient / Client (Last, First MI) ID Number Living Unit Date of Birth , Name – Individual Preparing This Form Name – Staff Contact Name / Telephone Number – Institution ANTICIPATED RECOMMENDED MEDICATION CATEGORY MEDICATION DOSAGE DAILY TOTAL DOSAGE RANGE RANGE Antipsychotic / Bipolar Agent Zyprexa oral tablet; Oral: 2.5 mg-50 mg with most doses in Zyprexa Zydis oral the 2.5 mg-20 mg range disintegrating tablet; IM: 5 mg-10 mg per dose, up to 3 doses Zyprexa Intramuscular 24 hours Injection; Long Acting Injectable: 150 mg-300 mg Zyprexa Relprevv IM every 2 weeks, Intramuscluar Injection 300 mg-405 mg IM every 4 weeks (olanzapine) The anticipated dosage range is to be individualized, may be above or below the recommended range but no medication will be administered without your informed and written consent. Recommended daily total dosage range of manufacturer, as stated in Physician’s Desk Reference (PDR) or another standard reference. This medication will be administered Orally Injection Other – Specify: 1. Reason for Use of Psychotropic Medication and Benefits Expected (note if this is ‘Off-Label’ Use) Include DSM-5 diagnosis or the diagnostic “working hypothesis.” 2. -

Aerogen Nebulizer)
Page 1 of Date of Original: 11/15/18 Date of Review: Date of Revision: 9/4/19 Respiratory Therapy POLICY & PROCEDURE MANUAL RIBAVIRIN ADMINISTRATION (Aerogen nebulizer) PURPOSE: 1. Standardize the administration and delivery of ribavirin by aerosol 2. To protect the patient and caregivers by standardizing precautions for ribavirin delivery POLICY: The patient room must be set up according to St Jude’s Environmental Health and Safety policy prior to the administration of Ribavirin. Room preparation is to be done by the nursing staff. *See procedure section below https://home.stjude.org/ehs/Pages/ribavirin-precautions.aspx • Negative pressure room is mandatory for Ribavirin administration. • Preparation of the room is the responsibility of the nursing staff. • Ribavirin will be delivered from pharmacy in primed Aerogen syringe set appropriate for use on the MedFusion 3500 syringe pump. • The syringe pumps will be used, maintained, and stored by the respiratory therapy department. • The PPE cart is the shared responsibility of the respiratory therapist who administers the first dose of ribavirin and the nursing coordinator for that area. • Proper sign including contact precautions and STOP will be shared responsibility of the nursing and respiratory care. • All instances of spilled or leaked ribavirin must be documented in the event reporting system. • Ribavirin may be delivered to a patient during mechanical ventilation in a very specific set of circumstances. The final decision on the use of ribavirin is at the discretion of the ICU physician on service. EQUIPMENT: • Negative pressure room • DeMistifier 2000 HEPA filtration system. • DeMistifier negative pressure bed enclosure with canopy. • MedFusion 3500 Syringe pump • Flowmeter • Aerogen vibrating mesh nebulizer • Delivery Supplies (O2 tubing, O2 reducer, large bore tubing, T adaptor, and aerosol face mask) • Prescribed medication • STOP sign for the door during drug administration PPE • N95 or Positive Air Pressure mask required • Chemo resistant Gown • Double Gloves • Shoe covers PROCEDURE: 1. -

Product Monograph
PRODUCT MONOGRAPH Pr SANDOZ ONDANSETRON ODT Ondansetron Orally Disintegrating Tablets 4 mg and 8 mg ondansetron Manufacturer Standard Pr SANDOZ ONDANSETRON Ondansetron Tablets 4 mg and 8 mg ondansetron (as ondansetron hydrochloride dihydrate) Manufacturer Standard PrONDANSETRON INJECTION USP (ondansetron hydrochloride dihydrate) 2 mg/mL PrONDANSETRON HYDROCHLORIDE DIHYDRATE INJECTION Ondansetron Injection Manufacturer Standard 2 mg/mL Ondansetron (as ondansetron hydrochloride dihydrate) for injection Antiemetic (5-HT3-receptor antagonist) Sandoz Canada Inc. Date of Preparation: 145 Jules-Léger September 26, 2016 Boucherville, Québec, Canada J4B 7K8 Control No.: 198381 Ondansetron by Sandoz Page 1 of 43 Table of Contents PART I: HEALTH PROFESSIONAL INFORMATION .......................................................... 3 SUMMARY PRODUCT INFORMATION ................................................................................. 3 INDICATIONS AND CLINICAL USE ....................................................................................... 4 CONTRAINDICATIONS ............................................................................................................ 4 WARNINGS AND PRECAUTIONS .......................................................................................... 5 ADVERSE REACTIONS ............................................................................................................ 7 DRUG INTERACTIONS ............................................................................................................ -

Technical Considerations for Pen, Jet, and Related Injectors Intended for Use with Drugs and Biological Products
Guidance for Industry and FDA Staff: Technical Considerations for Pen, Jet, and Related Injectors Intended for Use with Drugs and Biological Products Additional copies are available from: Office of Combination Products Office of Special Medical Programs Office of the Commissioner Food and Drug Administration 10903 New Hampshire Avenue, WO-32 Hub 5129 Silver Spring, MD 20993 (Tel) 301-796-8930 (Fax) 301-796-8619 http://www.fda.gov/CombinationProducts/default.htm This document finalizes the draft guidance issued in April 2009. For questions regarding this document, contact the Office of Combination Products at [email protected] or Patricia Y. Love, MD at 301-796-8933 or [email protected] U.S. Department of Health and Human Services Food and Drug Administration Center for Devices and Radiological Health, Center for Drug Evaluation Research, Center for Biologics Evaluation and Research, and Office of Combination Products in the Office of the Commissioner June 2013 Contains Nonbinding Recommendations Table of Contents INTRODUCTION.....................................................................................................................3 BACKGROUND .......................................................................................................................4 SECTION I: SCIENTIFIC AND TECHNICAL CONSIDERATIONS.............................5 A. INJECTOR DESCRIPTION .............................................................................................5 B. DESIGN FEATURES.........................................................................................................9