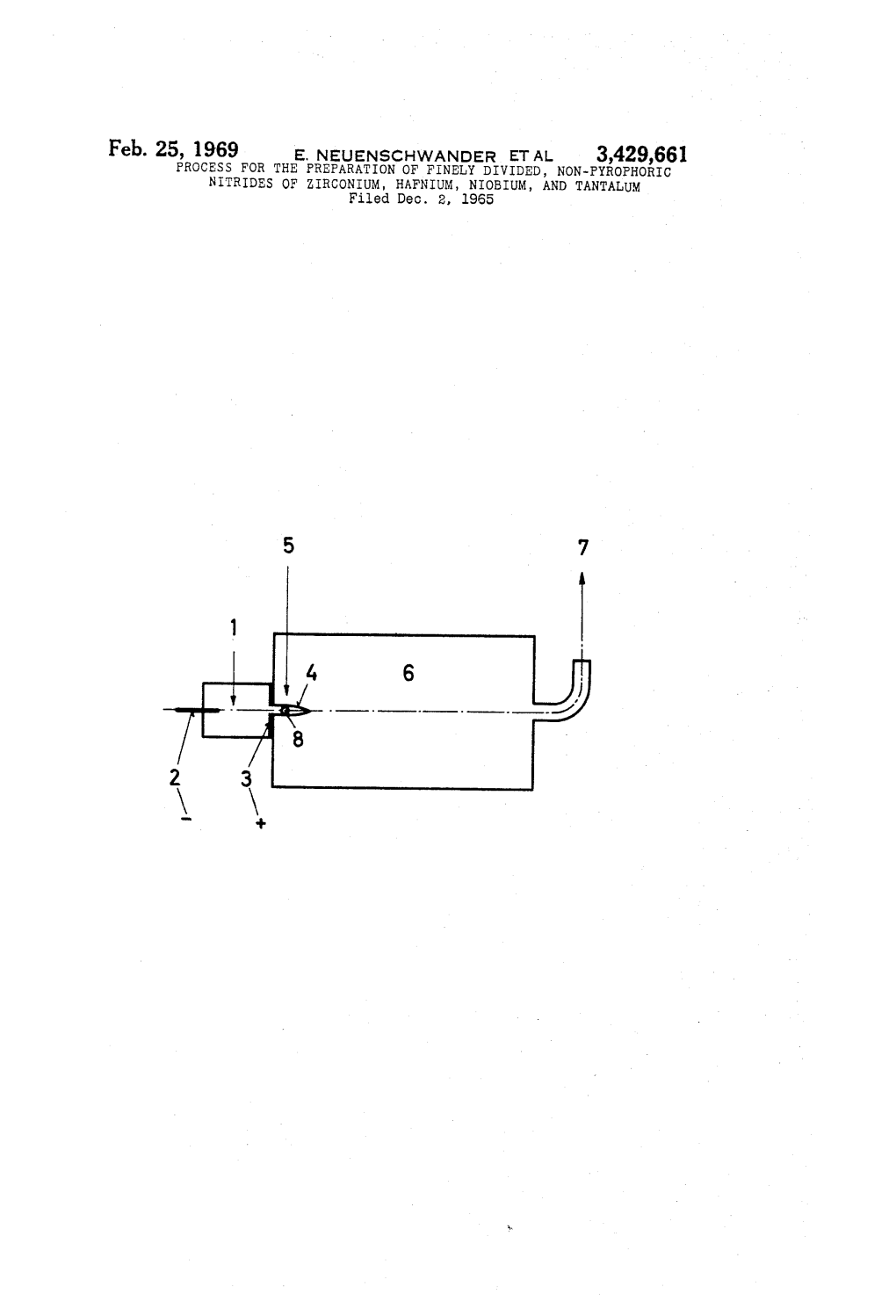
Feb. 25, 1969 E. NEUENSCHWANDER ET AL 3,429,661
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Thermodynamic Routes to Novel Metastable Nitrogen-Rich Nitrides
Article pubs.acs.org/cm Thermodynamic Routes to Novel Metastable Nitrogen-Rich Nitrides Wenhao Sun,†,‡ Aaron Holder,§,⊥ Bernardo Orvañanos,‡ Elisabetta Arca,§ Andriy Zakutayev,§ Stephan Lany,§ and Gerbrand Ceder*,†,‡,∥ † Materials Sciences Division, Lawrence Berkeley National Laboratory, Berkeley, California 94720, United States ‡ Department of Materials Science and Engineering, Massachusetts Institute of Technology, Cambridge, Massachusetts 02139, United States § National Renewable Energy Laboratory, Golden, Colorado 80401, United States ∥ Department of Materials Science and Engineering, Univeristy of California, Berkeley, California 94720, United States ⊥ Department of Chemical and Biological Engineering, Univeristy of Colorado, Boulder, Colorado 80309, United States *S Supporting Information ABSTRACT: Compared to oxides, the nitrides are relatively unexplored, making them a promising chemical space for novel materials discovery. Of particular interest are nitrogen-rich nitrides, which often possess useful semiconducting properties for electronic and optoelectronic applications. However, such nitrogen-rich compounds are generally metastable, and the lack of a guiding theory for their synthesis has limited their exploration. Here, we review the remarkable metastability of observed nitrides, and examine the thermodynamics of how reactive nitrogen precursors can stabilize metastable nitrogen-rich compositions during materials synthesis. We map these thermodynamic strategies onto a predictive computational search, training a data-mined -

Ultra-Thin Niobium Nitride Films for Hot Electron Bolometer and Thz Applications
THESIS FOR THE DEGREE OF LICENTIATE OF ENGINEERING Ultra-thin Niobium Nitride Films for Hot Electron Bolometer and THz Applications SASCHA KRAUSE Group for Advanced Receiver Development Department of Earth and Space Science CHALMERS UNIVERSITY OF TECHNOLOGY Gothenburg, Sweden 2016 i Ultra-thin Niobium Nitride Films for Hot Electron Bolometer and THz Applications SASCHA KRAUSE © SASCHA KRAUSE, 2016. Thesis for the degree of Licentiate of Engineering Group for Advanced Receiver Development Department of Earth and Space Science Chalmers University of Technology SE-412 96 Gothenburg Sweden Telephone + 46 (0)31-772 1000 Cover: Upper left corner: The Atacama Path Finder Experiment representing the exploration of space in the submillimeter and THz frequency range, upper right corner: HRTEM image of ultra-thin NbN film that is grown onto a GaN buffer-layer in an epitaxial manner, lower left corner: SEM image of the employed HEB used for measuring the phonon escape time, lower right corner: Resistance versus temperature behavior of the investigated ultra-thin NbN films which were grown onto different buffer-layers and substrates. [Printed by Chalmers Repro Service] Gothenburg, 2016 ii Ultra-thin Niobium Nitride Films for Hot Electron Bolometer and THz Applications SASCHA KRAUSE Group for Advanced Receiver Development Department of Earth and Space Science Chalmers University of Technology Gothenburg, Sweden 2016 Abstract The part of the electromagnetic spectrum between microwaves and infrared, also known as the terahertz band, is of particular interest for radio astronomy. The radiation intensity of the cold universe peaks at this frequency band, thus defining the demand on sensitive low-noise instruments in this particular frequency range. -

Development of a Nbn Deposition Process for Superconducting Quantum Sensors
1 Development of a NbN Deposition Process for Superconducting Quantum Sensors D. M. Glowacka, D. J. Goldie, S. Withington, H. Muhammad, G. Yassin , and B. K. Tan occurs with the addition of the reactive gas during sputtering, Abstract— We have carried out a detailed programme to which in turn affects stoichiometry. It is very important to explore the superconducting characteristics of reactive DC- quantify the location of the best operating point, and this magnetron sputtered NbN. The basic principle is to ignite a requires a careful study of the effects of deposition conditions. plasma using argon, and then to introduce a small additional Thakoor et al. [2] found that high-Tc NbN films were nitrogen flow to achieve the nitridation of a Nb target. Subsequent sputtering leads to the deposition of NbN onto the deposited at the point where the total sputter pressure started host substrate. The characteristics of a sputtered film depend on to increase rapidly with increasing nitrogen flow. In this paper, a number of parameters: argon pressure, nitrogen flow rate and we outline work that we have done to create a high-yield time-evolution profile, substrate material, etc. Crucially, the highly-reproducible deposition process for high-Tc NbN. We hysteresis in the target voltage as a function of the nitrogen flow also briefly describe work on realising and measuring can be used to provide a highly effective monitor of nitrogen resonators of the kind that would be suitable for a KID array consumption during the reactive process. By studying these dependencies we have been able to achieve highly reproducible operating at 3.5 K in an easy-to-use closed-cycle refrigerator. -

Synthesis and Photoelectrochemical Characterization of Tantalum Oxide Nanoparticles and Their Nitrogen Derivatives
University of Nevada, Reno Synthesis and Photoelectrochemical Characterization of Tantalum Oxide Nanoparticles and their Nitrogen Derivatives A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Chemical engineering by Vijay Khanal Dr. Vaidyanathan (Ravi) Subramanian – Advisor August 2019 The Graduate School We recommend that the dissertation prepared under our supervision by VIJAY KHANAL Entitled Synthesis and Photoelectrochemical Characterization of Tantalum Oxide Nanoparticles and their nitrogen Derivatives Be accepted in partial fulfillment of the Requirements for the degree of DOCTOR OF PHILOSOPHY Vaidyanathan (Ravi) Subramanian, Ph.D., Advisor Dhanesh Chandra, Ph.D., Committee Member Alan Fuchs, Ph.D., Committee Member Victor Vasquez, Ph.D., Committee Member Mario A. Alpuche, Ph.D. Graduate School Representative David W. Zeh, Ph.D., Dean, Graduate School August 2019 i ABSTRACT This work presents a surfactant-mediated approach for the synthesis of Ta2O5 nanopar- 2 1 2 1 ticles with a very high surface area (41 m g− vs. 1.6 m g− for commercial equivalent), and their subsequent nitridation to yield two distinct nitrogen derivatives: TaON and Ta3N5. Excellent photocatalytic efficiency of Ta2O5 toward photodegradation of methy- lene blue and NOx removal is reported. Ta3N5 shows significant photoconversion of methylene blue attributable to its lowered bandgap and high adsorption capability of MB molecules on its surface. A new method for simplifying the existing synthesis protocols of single phase Tantalum oxynitride (TaON) was devised. Series of photoelectrochemical characterization shows that the newly synthesized TaON can significantly enhance the photoassisted charge generation, separation, and transportation compared to the parent oxide (Ta2O5). -

Novel Semiconductor-Superconductor Structure Features Versatile Gallium Nitride 8 March 2018, by Tom Fleischman
Novel semiconductor-superconductor structure features versatile gallium nitride 8 March 2018, by Tom Fleischman niobium nitride (NbN), a proven superconductor material used in quantum communications, astronomy and a host of other applications. The group's paper, "GaN/NbN Epitaxial Semiconductor/Superconductor Heterostructures," is being published online March 8 in Nature. Former postdoctoral researcher Rusen Yan and current postdoc Guru Khalsa are co-lead authors. Other key contributors were Grace Xing, the Bandgap, lattice constant, crystallinity and Richard Lundquist Sesquicentennial Professor in superconductivity in epitaxial NbNx on SiC. a, Bandgap ECE and MSE, and David Muller, the Samuel B. versus lattice constant for select nitride semiconductors as well as for SiC. b, Cross-section HAADFSTEM Eckert Professor of Engineering in the Department images in black/white (left) and false-colour (right) of of Applied and Engineering Physics. 5-nm NbNx grown on an SiC substrate with a AlN capping layer. c, Resistance versus temperature The method for combining the two materials – (normalized to the resistance at 16K), showing the molecular beam epitaxy (MBE), essentially spray superconducting phase transition of 5-nm (red) and painting of gallium and nitrogen atoms onto the 35-nm (blue) . Credit: Nature (2018). DOI: NbN in a vacuum environment – creates an 10.1038/nature25768 extremely clean interface and is key to the success of the novel structure. This advance, the group says, opens up a range of Silicon has been the semiconductor material of possibilities that can now combine the macroscopic choice for electronics pretty much since the quantum effects of superconductors with the rich transistor effect was first observed and identified electronic and photonic properties of group III- nearly 80 years ago. -

Discovery of Superconductivity in Hard Hexagonal Ε-Nbn
BNL-111904-2016-JA Discovery of Superconductivity in Hard Hexagonal ε-NbN Yongtao Zou1,2, Xintong Qi3, Cheng Zhang4, Shuailing Ma1, Wei Zhang5, Ying Li2, Ting Chen3, Xuebing Wang3, Zhiqiang Chen2, David Welch4,6, Pinwen Zhu1, Bingbing Liu1, Qiang Li4, Tian Cui1 & Baosheng Li2 1State Key Laboratory of Superhard Materials, College of Physics, Jilin University, Changchun, 130012, China. 2Mineral Physics Institute, State University of New York, Stony Brook, N.Y. 11794, United States. 3Department of Geosciences, State University of New York, Stony Brook, N.Y. 11794, United States. 4Condensed Matter Physics and Materials Science Department, Brookhaven National Laboratory, Upton, N.Y. 11973, United States. 5School of Science, Southwest University of Science and Technology, Mianyang, Sichuan 621010, China. 6Department of Materials Science and Engineering, State University of New York, Stony Brook, N.Y. 11794, United States. *Correspondence and requests for materials should be addressed to Y. Z. (E-mail: [email protected] ), Y. L . (E-mail:[email protected]) **The current ultrasonic, magnetization and electrical resistivity measurements were performed in the U. S. when Yongtao Zou was working at the State University of New York (Stony Brook). Since the discovery of superconductivity in boron-doped diamond with a critical temperature (TC) near 4 K, great interest has been attracted in hard superconductors such as transition-metal nitrides and carbides. Here we report the new discovery of superconductivity in polycrystalline hexagonal ε-NbN synthesized at high pressure and high temperature. Direct magnetization and electrical resistivity measurements demonstrate that the superconductivity in bulk polycrystalline hexagonal ε-NbN is below ~11.6 K, which is significantly higher than that for boron-doped diamond. -

PVD Material Listing
P. O. Box 639 NL - 5550 AP Valkenswaard Tel: +31 (0)40 204 69 31 Fergutec Fax: +31 (0)40 201 39 81 E - mail: [email protected] PVD Material Listing Pure Metals Aluminum, Al Antimony, Sb Beryllium, Be Bismuth, Bi Boron, B Cadmium, Cd Calcium, Ca Carbon, C Cerium, Ce Chromium, Cr Cobalt, Co Copper, Cu Erbium, Er Gadolinium, Gd Gallium, Ga Germanium, Ge Gold, Au Hafnium, Hf Indium, In Iridium, Ir Iron, Fe Lanthanum, La Lead, Pb Magnesium, Mg Manganese, Mn Molybdenum, Mo Neodymium, Nd Nickel, Ni Niobium, Nb Osmium, Os Palladium, Pd Platinum, Pt Praseodymium, Pr Rhenium, Re Rhodium, Rh Ruthenium, Ru Samarium, Sm Selenium, Se Silicon, Si Silver, Ag Tantalum, Ta Fergutec b.v. P.O. Box 639, NL - 5550 AP Valkenswaard Heistraat 64, NL - 5554 ER Valkenswaard Bankaccount 45.80.36.714 ABN - AMRO Valkenswaard C.o.C. Eindhoven no. 17098554 VAT - ID NL8095.60.185.B01 The Standard Terms and Conditions, lodged at the Chamber of Commerce in Eindhoven, are applicable to all transactions. Tellurium, Te Terbium, Tb Tin, Sn Titanium, Ti Tungsten, W Vanadium, V Ytterbium, Yb Yttrium, Y Zinc, Zn Zirconium, Zr Precious Metals Gold Antimony, Au/Sb Gold Arsenic, Au/As Gold Boron, Au/B Gold Copper, Au/Cu Gold Germanium, Au/Ge Gold Nickel, Au/Ni Gold Nickel Indium, Au/Ni/In Gold Palladium, Au/Pd Gold Phosphorus, Au/P Gold Silicon, Au/Si Gold Silver Platinum, Au/Ag/Pt Gold Tantalum, Au/Ta Gold Tin, Au/Sn Gold Zinc, Au/Zn Palladium Lithium, Pd/Li Palladium Manganese, Pd/Mn Palladium Nickel, Pd/Ni Platinum Palladium, Pt/Pd Palladium Rhenium, Pd/Re Platinum Rhodium, -

Ligand-Based Reduction of CO2 to CO Mediated by an Anionic Niobium Nitride Complex
Ligand-Based Reduction of CO2 to CO Mediated by an Anionic Niobium Nitride Complex The MIT Faculty has made this article openly available. Please share how this access benefits you. Your story matters. Citation Silvia, Jared S., and Christopher C. Cummins. “Ligand-Based Reduction of CO2 to CO Mediated by an Anionic Niobium Nitride Complex.” Journal of the American Chemical Society 132.7 (2010) : 2169-2171. As Published http://dx.doi.org/10.1021/ja910445r Publisher American Chemical Society Version Author's final manuscript Citable link http://hdl.handle.net/1721.1/65162 Terms of Use Article is made available in accordance with the publisher's policy and may be subject to US copyright law. Please refer to the publisher's site for terms of use. Ligand-Based Reduction of CO2 to CO Mediated by an Anionic Niobium Nitride Complex Jared S. Silvia and Christopher C. Cummins Department of Chemistry, Massachusetts Institute of Technology, Cambridge, Massachusetts, 02139 RECEIVED DATE (automatically inserted by publisher); E-mail: [email protected] There are several motivations for producing chemicals from Scheme 1 CO2 whenever possible, and in particular, CO is a promising target being both a versatile chemical precursor and a fuel.1 Reducing metal complexes capable of O-atom abstraction from CO2 typically lead to the formation of strong metal- oxygen bonds that represent a challenge to catalytic turnover.2 In a special case for which the binding of CO2 evidently involves insertion into a Cu–B linkage, catalytic turnover producing CO was accomplished by taking advantage of the ultimate delivery of oxygen into a stable B–O–B reservoir.3 This is to be compared with electrocatalytic methods for CO2 reduction to CO in systems that likely involve a direct interaction between CO2 and the metal center at some point in the catalytic cycle.4 Another commonly observed trend in metal-mediated CO2 reduction is disproportionation reactivity 2– leading to the formation of CO and CO3 , highlighted by the reaction of Li2[W(CO)5] with CO2 to give W(CO)6 and 5 Li2CO3. -

The Role of Zirconium in Microalloyed Steels
The Role of Zirconium in Microalloyed Steels T N Baker Department of Mechanical and Aerospace Engineering The University of Strathclyde, Glasgow G1 1XJ, UK. E-mail:[email protected]. Abstract Recently there has been a renewed interest in the addition of zirconium to microalloyed steels. It has been used since the early 1920’s, but has never been universally employed, as have niobium, titanium or vanadium. The functions of zirconium in steelmaking are associated with a strong chemical affinity, in decreasing order, for oxygen, nitrogen, sulphur and carbon. Historically, the main use of additions of zirconium to steel was for combination preferentially with sulphur and so avoid the formation of manganese sulphide, known to have a deleterious influence of the impact toughness of wrought and welded steel. Modern steelmaking techniques have also raised the possibility that zirconium additions can reduce the austenite grain size and increase dispersion strengthening, due to precipitation of zirconium carbonitrides, or in high nitrogen vanadium-zirconium steels, vanadium nitride. This review gathers information on the compounds of zirconium identified in steels together with crystallographic data and solubility equations. Also brief accounts of the role of sulphides and particles in general on austenite grain size control and toughness are included. Keywords: Zirconium compounds, mechanical properties, impact, microstructure 1 1 Introduction The term microalloying, as applied to steels, is generally accepted as emanating from the paper by Beiser1 published in 1959, which reported the results of small additions of niobium to commercial heats of a carbon steel. However, it has not been recognized that microalloying as such, first occurred some 35 years earlier, when small additions of zirconium were added to plain carbon steels, and the effects reported by Feild2 and by Beckett3. -

This Thesis Has Been Submitted in Fulfilment of the Requirements for a Postgraduate Degree (E.G
This thesis has been submitted in fulfilment of the requirements for a postgraduate degree (e.g. PhD, MPhil, DClinPsychol) at the University of Edinburgh. Please note the following terms and conditions of use: This work is protected by copyright and other intellectual property rights, which are retained by the thesis author, unless otherwise stated. A copy can be downloaded for personal non-commercial research or study, without prior permission or charge. This thesis cannot be reproduced or quoted extensively from without first obtaining permission in writing from the author. The content must not be changed in any way or sold commercially in any format or medium without the formal permission of the author. When referring to this work, full bibliographic details including the author, title, awarding institution and date of the thesis must be given. Development of a High Temperature Sensor Suitable for Post-Processed Integration with Electronics Aleksandr Tabasnikov A thesis submitted for the degree of Doctor of Philosophy The University of Edinburgh 2016 Declaration of originality I declare that this thesis has been composed by me, the work is my own, and it has not been submitted for any other degree or professional qualification. Research recorded was carried out by me except as specified below: X-ray diffraction measurement results obtained using X-ray diffractometer Siemens D5000 were collected by Richard Yongqing Fu and his students Jimmy Zhou and Chao Zhao at the University of West of Scotland. Measurement results are analysed in section 4.4.6 of Chapter 4 and have been jointly published in 2014 (DOIs: 10.1109/ICMTS.2014.6841491). -

Carbides and Nitrides of Zirconium and Hafnium
materials Review Carbides and Nitrides of Zirconium and Hafnium Sergey V. Ushakov 1,* , Alexandra Navrotsky 1,* , Qi-Jun Hong 2,* and Axel van de Walle 2,* 1 Peter A. Rock Thermochemistry Laboratory and NEAT ORU, University of California at Davis, Davis, CA 95616, USA 2 School of Engineering, Brown University, Providence, RI 02912, USA * Correspondence: [email protected] (S.V.U.); [email protected] (A.N.); [email protected] (Q.-J.H.); [email protected] (A.v.d.W.) Received: 6 August 2019; Accepted: 22 August 2019; Published: 26 August 2019 Abstract: Among transition metal carbides and nitrides, zirconium, and hafnium compounds are the most stable and have the highest melting temperatures. Here we review published data on phases and phase equilibria in Hf-Zr-C-N-O system, from experiment and ab initio computations with focus on rocksalt Zr and Hf carbides and nitrides, their solid solutions and oxygen solubility limits. The systematic experimental studies on phase equilibria and thermodynamics were performed mainly 40–60 years ago, mostly for binary systems of Zr and Hf with C and N. Since then, synthesis of several oxynitrides was reported in the fluorite-derivative type of structures, of orthorhombic and cubic higher nitrides Zr3N4 and Hf3N4. An ever-increasing stream of data is provided by ab initio computations, and one of the testable predictions is that the rocksalt HfC0.75N0.22 phase would have the highest known melting temperature. Experimental data on melting temperatures of hafnium carbonitrides are absent, but minimum in heat capacity and maximum in hardness were reported for Hf(C,N) solid solutions. -

Surface Modification of G-C3N4 by Hydrazine: Simple Way for Noble-Metal Free Hydrogen Evolution Catalysts
Surface modification of g-C3N4 by hydrazine: Simple way for noble-metal free hydrogen evolution catalysts Item Type Article Authors Chen, Yin; Lin, Bin; Wang, Hong; Yang, Yong; Zhu, Haibo; Yu, Weili; Basset, Jean-Marie Citation Surface modification of g-C3N4 by hydrazine: Simple way for noble-metal free hydrogen evolution catalysts 2015 Chemical Engineering Journal Eprint version Post-print DOI 10.1016/j.cej.2015.10.080 Publisher Elsevier BV Journal Chemical Engineering Journal Rights NOTICE: this is the author’s version of a work that was accepted for publication in Chemical Engineering Journal. Changes resulting from the publishing process, such as peer review, editing, corrections, structural formatting, and other quality control mechanisms may not be reflected in this document. Changes may have been made to this work since it was submitted for publication. A definitive version was subsequently published in Chemical Engineering Journal, 2 November 2015. DOI: 10.1016/ j.cej.2015.10.080 Download date 03/10/2021 23:51:44 Link to Item http://hdl.handle.net/10754/581797 Accepted Manuscript Surface modification of g-C3N4 by hydrazine: Simple way for noble-metal free hydrogen evolution catalysts Yin Chen, Bin Lin, Hong Wang, Yong Yang, Haibo Zhu, Weili Yu, Jean-marie Basset PII: S1385-8947(15)01491-6 DOI: http://dx.doi.org/10.1016/j.cej.2015.10.080 Reference: CEJ 14356 To appear in: Chemical Engineering Journal Received Date: 18 June 2015 Revised Date: 9 October 2015 Accepted Date: 26 October 2015 Please cite this article as: Y. Chen, B. Lin, H. Wang, Y.