The Development and Investigation of an Audio Lure for Improved Possum (Trichosurus Vulpecula) Monitoring and Control in New Zealand
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Endangered and Threatened Species
DEPARTMENT OF NATURAL RESOURCES 315 NR27 Chapter NR 27 ENDANGERED AND THREATENED SPECIES NR 27.01 Definitions NR 27 .05 Permits for endangered and NR 27.02 Scope end applicability threatened species NR 27.03 Department list NR 27 .06 Exceptions to permit require NR 27.04 Revision of Wisconsin endan ments gered and threatened species NR 27.07 Severability lists Note: Chapter NR 27 es it existed on September 30, 1979 was repealed and a new chapter NR 27 was created effective October 1, 1979. NR 27.01 Definitions. As used in this chapter: (1) "Department" means the Wisconsin department of natural re sources. (2) "Department list" means the U.S. list of endangered and threatened foreign and native species, and the Wisconsin list of endan gered and threatened species. (3) "ENS" means the Office of Endangered and Nongame Species, Department of Natural Resources, Box 7921, Madison, WI 53707. (4) "Take" means shooting, shooting at, pursuing, hunting, catching or killing any wild animal; or the cutting, rooting up, severing, injuring, destroying, removing, or carrying away any wild plant. Hlotory: Cr. Register, September, 1979, No. 285, eff. 10-1-79. NR 27.02 Scope and applicability. This chapter contains rules nec essary to implement s. 29.415, Stats., and operate in conjunction with that statute to govern the taking, transportation, possession, processing or sale of any wild animal or wild plant specified by the department's lists of endangered and threatened wild animals and wild plants. Hlotory: Cr. Register, September, 1979, No. 285, eff. 10-1-79. NR 27.03 Department list. -

Engelsk Register
Danske navne på alverdens FUGLE ENGELSK REGISTER 1 Bearbejdning af paginering og sortering af registret er foretaget ved hjælp af Microsoft Excel, hvor det har været nødvendigt at indlede sidehenvisningerne med et bogstav og eventuelt 0 for siderne 1 til 99. Tallet efter bindestregen giver artens rækkefølge på siden. -

Hemideina Crassidens
UNIVERSITÉ DU QUÉBEC À MONTRÉAL LE MAINTIEN DE STRATÉGIES ALTERNATIVES CHEZ LE WELLINGTON TREE WETA (HEM/DE/NA CRASSIDENS): TESTER LES PRÉDICTIONS DE L'HYPOTHÈSE DE POLYMORPHISME GÉNÉTIQUE MÉMOIRE PRÉSENTÉ COMME EXIGENCE PARTIELLE DE LA MAÎTRISE EN BIOLOGIE PAR SARAHNASON JANVIER 2019 UNIVERSITÉ DU QUÉBEC À MONTRÉAL Service des bibliothèques Avertissement La diffusion de ce mémoire se fait dans le respect des droits de son auteur, qui a signé le formulaire Autorisation de reproduire et de diffuser un travail de recherche de cycles supérieurs (SDU-522 - Rév.07-2011). Cette autorisation stipule que «conformément à l'article 11 du Règlement no 8 des études de cycles supérieurs, [l'auteur] concède à l'Université du Québec à Montréal une licence non exclusiye d'utilisation et de publication de la totalité ou d'une partie importante de [son] travail de recherche pour des fins pédagogiques et non commerciales. Plus précisément, [l'auteur] autorise l'Université du Québec à Montréal à reproduire, diffuser, prêter, distribuer ou vendre des copies de [son] travail de recherche à des fins non commerciales sur quelque support que ce soit, y compris l'Internet. Cette licence et cette autorisation n'entraînent pas une renonciation de [la] part [de l'auteur] à [ses] droits moraux ni à [ses] droits de propriété intellectuelle. Sauf entente contraire, [l'auteur] conserve la liberté de diffuser et de commercialiser ou non ce travail dont [il] possède un exemplaire.» UNIVERSITÉ DU QUÉBEC À MONTRÉAL THE MAINTENANCE OF ALTERNATIVE STRATEGIES IN WELLINGTON TREE WETA (HEMIDEINA CRASSJDENS): TESTING THE PREDICTIONS OF THE GENETIC POLYMORPHISM HYPOTHESIS MÉMOIRE PRESENTED IN FULFILLMENT OF MASTER'S IN BIOLOGY BY SARAHNASON JANUARY, 2019 REMERCIEMENTS 1 would like to thank my supervisor Dr. -

Male Tree Weta Are Attracted to Cuticular Scent Cues but Do Not Discriminate According to Sex Or Among Two Closely Related Species
Received: 26 April 2017 | Revised: 7 June 2017 | Accepted: 3 July 2017 DOI: 10.1111/eth.12652 RESEARCH PAPER Male tree weta are attracted to cuticular scent cues but do not discriminate according to sex or among two closely related species Priscilla M. Wehi1,2 | Adrian Monks2 | Mary Morgan-Richards1 1Institute of Agriculture & Environment, Massey University, Palmerston North, Abstract New Zealand Recognition of conspecifics is an essential precursor of successful mating. Where 2 Landcare Research, Dunedin, New Zealand related species coexist, species discrimination might be important, but because related Correspondence species are similar, species signal recognition may actually be low. Chemical cues such Priscilla M. Wehi, Landcare Research, Private as cuticular hydrocarbons (CHCs) are frequently used by insects to identify suitable Bag 1930 Dunedin, 9054 New Zealand. Email: [email protected] sexual partners. We predicted that New Zealand tree weta (Hemideina spp.), a genus Funding information of nocturnal ensiferan Orthoptera that live both allopatrically and sympatrically, use This work was supported by New Zealand chemical signals from either frass or CHCs to find mates. In a series of six laboratory Foundation for Research, Science and Technology contract number UOWX0501, trials using both H. thoracica and H. crassidens, we found that male tree weta, but not and Postdoctoral fellowship MAUX0905; female tree weta, occupied cavities primed with female cuticular cues more often than Rutherford Discovery Fellowship 14- LCR- 001 to PMW; and Massey University MURF cavities without. However, males did not discriminate between chemical cues of male funding “What limits a weta?” to MMR. and female conspecifics, or between conspecifics and heterospecifics. -

1 DEPARTMENT of the INTERIOR Fish and Wildlife
This document is scheduled to be published in the Federal Register on 08/04/2016 and available online at http://federalregister.gov/a/2016-17322, and on FDsys.gov DEPARTMENT OF THE INTERIOR Fish and Wildlife Service 50 CFR Part 17 [Docket No. FWS–R9–ES–2008–0063; 92300-1113-0000-9B] RIN 1018–AU62 Endangered and Threatened Wildlife and Plants; Amending the Formats of the Lists of Endangered and Threatened Wildlife and Plants AGENCY: Fish and Wildlife Service, Interior. ACTION: Final rule. SUMMARY: We, the U.S. Fish and Wildlife Service, amend the format of the Lists of Endangered and Threatened Wildlife and Plants (Lists) to reflect current practices and standards that will make the regulations and Lists easier to understand. The Lists, in the new format, are included in their entirety and have been updated to correct identified errors. 1 DATES: This rule is effective [INSERT DATE OF PUBLICATION IN THE FEDERAL REGISTER]. FOR FURTHER INFORMATION CONTACT: Don Morgan, Ecological Services Program, U.S. Fish and Wildlife Service, 5275 Leesburg Pike, Falls Church, VA, 22041; telephone 703– 358–2171. If you use a telecommunications device for the deaf (TDD), call the Federal Information Relay Service (FIRS) at 800–877–8339. SUPPLEMENTARY INFORMATION: Background The Lists of Endangered and Threatened Wildlife and Plants (Lists), found in title 50 of the Code of Federal Regulations (CFR) at 50 CFR 17.11 for wildlife and 50 CFR 17.12 for plants, contain the names of endangered species and threatened species officially listed pursuant to the Endangered Species Act of 1973, as amended (16 U.S.C. -

Factors Influencing Occupancy of Modified Artificial Refuges for Monitoring the Range-Restricted Banks Peninsula Tree Weta Hemideina Ricta (Anostostomatidae)
132 AvailableNew on-lineZealand at: Journal http://www.newzealandecology.org/nzje/ of Ecology, Vol. 38, No. 1, 2014 Factors influencing occupancy of modified artificial refuges for monitoring the range-restricted Banks Peninsula tree weta Hemideina ricta (Anostostomatidae) Mike H. Bowie1*, Warwick J. Allen1, Jill McCaw1 and Rachel van Heugten2 1Ecology Department, Agriculture and Life Sciences Division, PO Box 85084, Lincoln University, Lincoln 7647, New Zealand 2School of Biological Sciences, University of Canterbury, Private Bag 4800, Christchurch 8140, New Zealand *Author for correspondence (Email: [email protected]) Published online: 7 November 2013 Abstract: The use of non-destructive and non-invasive monitoring methods is often necessary for species of high conservation status. Developing monitoring methods to maximise numbers of individuals found is important, given that rare species can be difficult to locate. Artificial refuges called ‘weta motels’ have been used for monitoring tree weta (Orthoptera: Anostostomatidae) since 1992, but poor occupancy for Hemideina ricta and H. femorata necessitated an improved design and assessment of placement to encourage tree weta use. Modification to a basic design of weta motel was tested on New Zealand’s rarest tree weta, H. ricta, on Banks Peninsula, Canterbury, New Zealand. Possible lures such as peanut butter or frass from male and female H. ricta were placed in motels in an attempt to improve occupancy. We recorded high occupancy rates with an improved weta motel design and found that motels containing female frass had significantly higher levels of occupancy than controls, with the former reaching 80% occupation after 6 months. Weta motels were more likely to be used by tree weta in areas with low subcanopy density and patchy or little canopy cover, with H. -

Title 50 Part 17
U Title 50—Wildlife and Fisheries Ihe information of the reader. In the annual all other appropriate rules in Parts 17. 217 revision and compilation of this title, the through 227. and 402 still apply to that PART 17—ENDANGERED AND following information may be amended species. In addition, there may be other rules THREATENED WILDLIFE AND PLANTS without public notice: the spelling of species' in this title that relate to such wildlife, e.g.. names, historical range, footnotes, references pon-of-entry requirements. It is not intended to certain other applicable portions of this that the references m the "Special rules" title, synonyms, and more current names. In column list all the regulations of the two Subpart B—Lists any of these revised entries, neither the Services which might apply to the species or § 17.11 Endangered and threatened species, as defined in paragraph (b) of this to the regulations of other Federal agencies wildlife. section, nor its status may be changed without or Stale or local governments. (a) The list in this section contains the following the procedures of Part 424 of this (g) The listing of a particular taxon names of all species of wildlife which have title. includes all lower taxonomic units. For been determined by Ihe Services to be (e) The "historic range" indicates the example, the genus H\lobaies (gibbons) is Endangered or Threatened. It also contains known general distribution of the species or listed as Endangered throughout its entire the names of species of wildlife treated as subspecies as reported in the current scientific range (China. -

Macroinvertebrate Community Responses to Mammal Control
MACROINVERTEBRATE COMMUNITY RESPONSES TO MAMMAL CONTROL – EVIDENCE FOR TOP-DOWN TROPHIC EFFECTS BY OLIVIA EDITH VERGARA PARRA A thesis submitted to the Victoria University of Wellington in fulfilment of the requirements for the degree of Doctor of Philosophy in Conservation Biology Victoria University of Wellington 2018 Para mi sobrina Violeta Orellana Vergara y su sonrisa hermosa. Tu llegada remeció mi corazón de amor de una manera inimaginable. ¡Sueña en grande! ii Nothing in nature stands alone... (John Hunter 1786) iii iv ABSTRACT New Zealand’s invertebrates are characterised by extraordinary levels of endemism and a tendency toward gigantism, flightlessness and longevity. These characteristics have resulted in a high vulnerability to introduced mammals (i.e. possums, rats, mice, and stoats) which are not only a serious threat to these invertebrates, but have also altered food web interactions over the past two-hundred years. The establishment of fenced reserves and the aerial application of 1080 toxin are two methods of mammal control used in New Zealand to exclude and reduce introduced mammals, respectively. Responses of ground-dwelling invertebrates to mammal control, including a consideration of trophic cascades and their interactions, remain unclear. However, in this thesis, I aimed to investigate how changes in mammal communities inside and outside a fenced reserve (ZEALANDIA, Wellington) and before-and-after the application of 1080 in Aorangi Forest, influence the taxonomic and trophic abundance, body size and other traits of ground-dwelling invertebrates on the mainland of New Zealand. I also tested for effects of habitat variables (i.e. vegetation and elevation), fluctuations in predator populations (i.e. -

Orthoptera: Ensifera)?
Zootaxa 4291 (1): 001–033 ISSN 1175-5326 (print edition) http://www.mapress.com/j/zt/ Article ZOOTAXA Copyright © 2017 Magnolia Press ISSN 1175-5334 (online edition) https://doi.org/10.11646/zootaxa.4291.1.1 http://zoobank.org/urn:lsid:zoobank.org:pub:BD31B828-E7EF-46AD-B618-1BAAA2D63DBD Tackling an intractable problem: Can greater taxon sampling help resolve relationships within the Stenopelmatoidea (Orthoptera: Ensifera)? AMY G. VANDERGAST1,7, DAVID B. WEISSMAN2, DUSTIN A. WOOD3, DAVID C. F. RENTZ4, CORINNA S. BAZELET5 & NORIHIRO UESHIMA6 1U.S. Geological Survey, Western Ecological Research Center, San Diego Field Station, 4165 Spruance Road Suite 200, San Diego, CA 92101, USA. E-mail: [email protected] 2Department of Entomology, California Academy of Sciences, 55 Music Concourse Drive, San Francisco, CA 94118, USA. E-mail: [email protected] 3U.S. Geological Survey, Western Ecological Research Center, San Diego Field Station, 4165 Spruance Road Suite 200, San Diego, CA 92101, USA. E-mail: [email protected] 4School of Marine & Tropical Biology, James Cook University, Australia. E-mail: [email protected] 5Steinhardt Museum, Tel Aviv University, Department of Zoology, Sherman Building Rm. 403, Tel Aviv, Israel; Department of Conser- vation Ecology and Entomology, Stellenbosch University, Private Bag X1, Matieland 7602, South Africa. E-mail: [email protected] 61435-1 Kubocho, Matsusaka, Mie 515-0044, Japan. E-mail: [email protected] 7Corresponding Author Abstract The relationships among and within the families that comprise the orthopteran superfamily Stenopelmatoidea (suborder Ensifera) remain poorly understood. We developed a phylogenetic hypothesis based on Bayesian analysis of two nuclear ribosomal and one mitochondrial gene for 118 individuals (84 de novo and 34 from GenBank). -
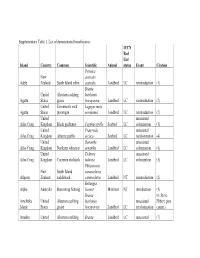
Supplementary Table 1. List of Demonstrated Beneficiaries
Supplementary Table 1. List of demonstrated beneficiaries. IUCN Red List Island Country Common Scientific Animal status Event Citation Petroica New australis Adele Zealand South Island robin australis Landbird LC reintroduction (1) Branta United Aleutian cackling hutchinsii Agattu States goose leucopareia Landbird LC reintroduction (2) United Evermann's rock Lagopus muta Agattu States ptarmigan evermanni Landbird LC reintroduction (2) United unassisted Ailsa Craig Kingdom Black guillemot Cepphus grylle Seabird LC colonization (3) United Fratercula unassisted Ailsa Craig Kingdom Atlantic puffin arctica Seabird LC recolonization (4) United Oenanthe unassisted Ailsa Craig Kingdom Northern wheatear oenanthe Landbird LC colonization (4) United Tadorna unassisted Ailsa Craig Kingdom Common shelduck tadorna Landbird LC colonization (3) Philesturnus New South Island carunculatus Allports Zealand saddleback carunculatus Landbird NT reintroduction (2) Bettongia Alpha Australia Burrowing bettong lesueur Mammal NT introduction (5) Branta (6; Steve Amchitka United Aleutian cackling hutchinsii unassisted Ebbert, pers. Island States goose leucopareia Landbird LC recolonization comm.) Amukta United Aleutian cackling Branta Landbird LC unassisted (7) IUCN Red List Island Country Common Scientific Animal status Event Citation States goose hutchinsii recolonization leucopareia Sally Amy Poncet, Island/Outer United Cinclodes unassisted unpublished Knob Kingdom Tussacbird antarcticus Landbird LC recolonization data Sally Amy Poncet, Island/Outer United unpublished -

Sex- and Season-Dependent Behaviour in a Flightless Insect, the Auckland Tree Weta (Hemideina Thoracica)
AvailableWehi et al.: on-line Seasonal at: http://www.newzealandecology.org/nzje/ behaviour patterns in weta 75 Sex- and season-dependent behaviour in a flightless insect, the Auckland tree weta (Hemideina thoracica) Priscilla M Wehi1,4*, Murray Jorgensen2 and Mary Morgan-Richards3 1Department of Biological Sciences, University of Waikato, Private Bag 3105, Hamilton 3240, New Zealand 2Department of Statistics, University of Waikato, Private Bag 3105, Hamilton 3240, New Zealand 3Institute of Natural Resources, Massey University, Private Bag 11222, Palmerston North, New Zealand 4Present address: CSAFE, University of Otago, P O Box 56, Dunedin, New Zealand *Author for correspondence (Email: [email protected]) Published online: 17 December 2012 Abstract: In a polygynous mating system, males frequently compete by locating and defending sites with resources essential to female survival and reproduction. We investigated seasonal changes in site occupancy in a sexually dimorphic, harem-forming insect, the Auckland tree weta (Hemideina thoracica). First we established artificial cavities as diurnal refuge cavities and potential harem guarding sites. We then examined cavity occupancy changes, and, based on our knowledge of prior occupants, determined sex-specific patterns of arrival, departure, and aggregation at a population level throughout the year. Both season and the sex of prior occupants influenced weta occupancy patterns. Most observations were of single females. However, both males and females moved into cavities previously occupied by a weta of the opposite sex more often than expected by chance alone. Females avoided cavities where other females were present, except during summer when most harems formed. In early summer, male and female tree weta previously living apart began co-habiting. -

Diet Selectivity in a Terrestrial Forest Invertebrate, the Auckland Tree Wētā, Across Three Habitat Zones
CORE Metadata, citation and similar papers at core.ac.uk Provided by Research Commons@Waikato Received: 11 September 2017 | Accepted: 4 December 2017 DOI: 10.1002/ece3.3763 ORIGINAL RESEARCH Diet selectivity in a terrestrial forest invertebrate, the Auckland tree wētā, across three habitat zones Matthew B. G. J. Brown1 | Chrissen E. C. Gemmill1 | Steven Miller2 | Priscilla M. Wehi3 1School of Science, University of Waikato, Hamilton, New Zealand Abstract 2Department of Mathematics and Insects are important but overlooked components of forest ecosystems in New Statistics, School of Computing and Zealand. For many insect species, information on foraging patterns and trophic rela- Mathematical Sciences, University of Waikato, Hamilton, New Zealand tionships is lacking. We examined diet composition and selectivity in a large- bodied 3Centre for Sustainability, University of Otago insect, the Auckland tree wētā Hemideina thoracica, in three habitat zones in a lowland and Manaaki Whenua Landcare Research, New Zealand forest. We asked whether H. thoracica selectively forage from available Dunedin, New Zealand plant food sources, and whether these choices were lipid- rich compared to nonpre- Correspondence ferred available plants. We also identified the proportion of invertebrates in their frass Priscilla M. Wehi, Manaaki Whenua Landcare Research, Dunedin, New Zealand. as a proxy for omnivory. From reconnaissance plot sampling, together with fecal frag- Email: [email protected] ment analysis, we report that more than 93% of individual tree wētā had eaten inver- Funding information tebrates before capture. Additionally, wētā in the highest elevation hillslope habitat University of Waikato; Royal Society of New zone consumed significantly fewer species of plants on average than wētā on the low- Zealand, Grant/Award Number: 14-LCR-001 elevation terrace habitat.