Desiccant Materials and Process for Use in Gas Fired Cooling and Dehumidification Equipment
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Peroxy Compounds Human Health and Ecological Draft Risk Assessment DP 455445, 455446
Peroxy Compounds Human Health and Ecological Draft Risk Assessment DP 455445, 455446 UNITED STATES ENVIRONMENTAL PROTECTION AGENCY WASHINGTON, D.C. 20460 OFFICE OF CHEMICAL SAFETY AND POLLUTION PREVENTION MEMORANDUM Date: March 11, 2020 SUBJECT: Registration Review Draft Risk Assessment for the Peroxy Compounds PC Code: 000595, 063201, 063604, 063607, DP Barcode: 455445, 455446 063209, 128860 Decision No: 558073, 558074 Docket No: EPA-HQ-OPP-2009-0546 Regulatory Action: Registration Review Case No: 6059, 4072, 5081 Risk Assessment Type: DRA CAS No: 7722-84-1, 79-21-0, 33734-57-5, 15630-89-4, 10058-23-8, 70693-62-8 TO: Kendall Ziner, Chemical Review Manager Rick Fehir, Ph.D., Team Lead Rose Kyprianou, Branch Chief Regulatory Management Branch (RMB) II Antimicrobials Division (7510P) Office of Pesticide Programs FROM: Andrew Byro, Ph.D., Chemist Kathryn Korthauer, Biologist Timothy Dole, Industrial Hygienist Deborah Burgin, Ph.D., DABT, Toxicologist Risk Assessment and Science Support Branch Antimicrobials Division (7510P) Office of Pesticide Programs THROUGH: Judy Facey, Ph.D., Human Health Risk Assessment Process Leader MP for JF Diana Hsieh, Ecological Risk Assessment Process Leader MP for DH Timothy Leighton, Senior Science Advisor MP for TL Laura Parsons, Associate Branch Chief Melissa Panger, Ph.D., Branch Chief Risk Assessment and Science Support Branch Antimicrobials Division (7510P) This document provides the draft human health and ecological risk assessment conducted in support of the antimicrobial use sites of the following peroxy compounds: hydrogen peroxide, peracetic acid, peroxyoctanoic acid, and sodium percarbonate. Page 1 of 74 Peroxy Compounds Human Health and Ecological Draft Risk Assessment DP 455445, 455446 Although the peroxymonosulfate compounds were included in the peroxy compounds Final Work Plan (FWP), they will not be included in this risk assessment. -

WO 2016/196440 Al 8 December 2016 (08.12.2016) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2016/196440 Al 8 December 2016 (08.12.2016) P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every A61P 3/04 (2006.01) A61K 33/40 (2006.01) kind of national protection available): AE, AG, AL, AM, A61P 9/10 (2006.01) A61K 38/44 (2006.01) AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, A61K 35/74 (2015.01) A61K 31/17 (2006.01) BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (21) International Application Number: HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, PCT/US20 16/034973 KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, (22) International Filing Date: MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, 3 1 May 2016 (3 1.05.2016) PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, (25) Filing Language: English TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (26) Publication Language: English (84) Designated States (unless otherwise indicated, for every (30) Priority Data: kind of regional protection available): ARIPO (BW, GH, 62/169,480 1 June 2015 (01 .06.2015) US GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, 62/327,283 25 April 2016 (25.04.2016) US TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, TJ, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, (71) Applicant: XENO BIOSCIENCES INC. -
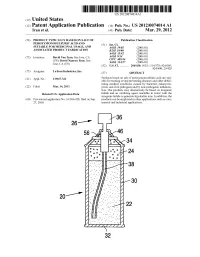
Us 2012/0074.014 A1 2 .. 1
US 2012O074O14A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2012/0074.014 A1 Tran et al. (43) Pub. Date: Mar. 29, 2012 (54) PRODUCT TYPICALLY BASED ON SALT OF Publication Classification PEROXYMONOSULFURIC ACID AND (51) Int. Cl SUITABLE FOR MEDICINAL USAGE, AND iBio/02 (2006.01) ASSOCATED PRODUCT FABRICATION B23P 19/00 (2006.01) A633/42 (2006.01) (75) Inventors: David Van Tran, San Jose, CA A6IR 9/14 (2006.01) (US); David Nguyen Tran, San CD7C 409/244 (2006.01) Jose, CA (US) A6II 3/327 (2006.01) s (52) U.S. Cl. ............. 206/438: 562/1; 514/578; 424/605; 424/400; 29/428 (73) Assignee: LuTran Industries, Inc. (57) ABSTRACT 21) Appl. No.: 13AO47,742 Products based on salt of peroxyperoxVmonosulfuric acid are suit (21) Appl. No 9 able for treating or/and preventing diseases and other debili tating medical conditions caused by bacterial, eukaryotic, (22) Filed: Mar. 14, 2011 prion, and viral pathogens and by non-pathogenic inflamma tion. The products may alternatively be based on inorganic Related U.S. Application Data halide and an oxidizing agent reactable in water with the inorganic halide to generate hypohalite ions. In addition, the (60) Provisional application No. 61/386,928, filed on Sep. products can be employed in other applications such as com 27, 2010. mercial and industrial applications. 5T. 2 .. 2. 1.4. J., Patent Application Publication Mar. 29, 2012 Sheet 1 of 2 US 2012/0074014 A1 cro Q-D 22 Fig. 2a Fig.2b Patent Application Publication Mar. 29, 2012 Sheet 2 of 2 US 2012/0074014 A1 90 92 US 2012/0074014 A1 Mar. -

Studies on Peroxymonosulfuric Acid Treatment for Totally Chlorine-Free Bleaching of Hardwoods Prehydrolysis-Kraft Pulps
Studies on Peroxymonosulfuric Acid Treatment for Totally Chlorine-free Bleaching of Hardwoods Prehydrolysis-kraft Pulps 著者 RIZALUDDIN Andri Taufick year 2016 その他のタイトル 広葉樹材前加水分解クラフトパルプの完全無塩素漂 白のためのモノ過硫酸処理に関する研究 学位授与大学 筑波大学 (University of Tsukuba) 学位授与年度 2015 報告番号 12102甲第7760号 URL http://hdl.handle.net/2241/00143908 Studies on Peroxymonosulfuric Acid Treatment for Totally Chlorine-free Bleaching of Hardwoods Prehydrolysis-kraft Pulps January 2016 Andri Taufick RIZALUDDIN Studies on Peroxymonosulfuric Acid Treatment for Totally Chlorine-free Bleaching of Hardwoods Prehydrolysis-kraft Pulps A Dissertation Submitted to The Graduate School of Life and Environmental Sciences, The University of Tsukuba In Partial Fulfillment on the Requirements For the Degree of Doctor of Philosophy in Bioresource Engineering (Doctoral Program in Appropriate Technology and Sciences for Sustainable Development) Andri Taufick RIZALUDDIN Table of Contents Chapter 1 Introduction ........................................................................................................... 1 1.1 Indonesian pulp and paper industry........................................................................... 1 1.2 Indonesia’s environmental policies regarding the pulp and paper industry .............. 2 1.3 Bleaching process .................................................................................................... 11 1.3.1 Oxygen bleaching .............................................................................................. 11 1.3.2 Chlorine dioxide bleaching -

Quality Performs
QUALITY PERFORMS. Monopersulfate Compound General Technical Attributes. QUALITY WORKS. MONOPERSULFATE COMPOUND PRODUCT INFORMATION What is OxoneTM? Applications* Oxone™ monopersulfate compound is a white, granular, n Swimming pool shock oxidizer free-flowing peroxygen that provides powerful non-chlorine n Printed wiring board microetchant oxidation for a wide variety of industrial and consumer uses. n Repulping aid for wet-strength resin destruction n Odor control agent in wastewater treatment The active ingredient of Oxone™ is potassium peroxymono- n Bleach component in denture cleanser and laundry sulfate, KHSO5, commonly known as potassium monoper- formulations sulfate, which is present as a component of a triple salt with n Disinfectant active ingredient n the formula 2KHSO5•KHSO4•K2SO4 (pentapotassium bis- Other uses, where its combination of powerful oxidation (peroxymonosulphate) bis(sulphate), [CAS 70693-62-8]). and relative safe handling properties are of value *As with any product, use of Oxone™ in a given application must be tested (including field testing, etc.) by the user in advance to determine suitability. The oxidizing power of Oxone™ is derived from its peracid chemistry; it is the first neutralization salt of peroxymonosulfuric acid H2SO5 (also known as Caro’s acid). Figure 2: Effect of pH on Oxone™ Solution Stability Standard Potential (3 wt% Solution at 32°C [90°F]) 110The standard electrode potential (E°) of KHSO5 is given by 2 the following half cell reaction: 100 20 21C (0F) – + – 0 90HSO5 + 2H + 2e- HSO4 + H2O E = 1.85V 1 0The thermodynamic potential is high enough for many 10 0room temperature oxidations, including: 5 3C (9F) 60 0 n Halide to active halogen pH 4.0 5.0 6.0 7.0 8.0 9.0 10.0 11.0 12.0 13.0 14.0 0n Oxidation of reduced sulfur and nitrogen compounds n Cyanide to cyanate 40 If a decomposition is associated with high temperature, n Epoxidation of olefins decomposition of the constituent salts of Oxone™ may 30n Baeyer-Villiger oxidation of ketones generate sulfuric acid, sulfur dioxide, or sulfur trioxide. -

Tailored Interfaces in Fiber-Reinforced Elastomers: a Surface Treatment Study on Optimized Load Coupling Via the Modified Fiber Bundle Debond Technique
polymers Article Tailored Interfaces in Fiber-Reinforced Elastomers: A Surface Treatment Study on Optimized Load Coupling via the Modified Fiber Bundle Debond Technique Julia Beter 1,* , Boris Maroh 1, Bernd Schrittesser 1 , Inge Mühlbacher 1, Thomas Griesser 2, Sandra Schlögl 1 , Peter Filipp Fuchs 1 and Gerald Pinter 3 1 Polymer Competence Center Leoben GmbH, Roseggerstrasse 12, 8700 Leoben, Austria; [email protected] (B.M.); [email protected] (B.S.); [email protected] (I.M.); [email protected] (S.S.); [email protected] (P.F.F.) 2 Chair of Chemistry of Polymeric Materials, Montanuniversitaet Leoben, Otto-Gloeckel Strasse 2, 8700 Leoben, Austria; [email protected] 3 Department of Polymer Engineering and Science, Montanuniversitaet Leoben, Otto-Gloeckel Strasse 2, 8700 Leoben, Austria; [email protected] * Correspondence: [email protected]; Tel.: +43-3842-42962-31 Abstract: The interface between the reinforcement and surrounding matrix in a fibrous composite is decisive and critical for maintaining component performance, durability, and mechanical structure properties for load coupling assessment, especially for highly flexible composite materials. The clear trend towards tailored solutions reveals that an in-depth knowledge on surface treating methods to enhance the fiber–matrix interfacial interaction and adhesion properties for an optimized load transfer needs to be ensured. This research aims to quantify the effect of several surface treatments for glass fibers applied in endless fiber-reinforced elastomers with pronounced high deformations. Due to this, the glass fiber surface is directly modified with selected sizings, using a wet chemical Citation: Beter, J.; Maroh, B.; treatment, and characterized according to chemical and mechanical aspects. -

Jeffrey E. Lucius U.S. Geological Survey This Report Is Preliminary
U. S. DEPARTMENT OF THE INTERIOR GEOLOGICAL SURVEY PHYSICAL AND CHEMICAL PROPERTIES AND HEALTH EFFECTS OF THIRTY-THREE TOXIC ORGANIC CHEMICALS Jeffrey E. Lucius U.S. Geological Survey Open-File Report 87-428 August 1987 This report is preliminary and has not been reviewed for conformity with U.S. Geological Survey editorial standards. Any use of trade names is for descriptive purposes only and does not imply endorsement by the U.S. Geological Survey. CONTENTS Page Introduction 4 The Properties 6 Abbreviations 13 Conversion Factors 16 Summary Tables 17 Acetic acid 27 Acetone 31 Benzene 34 Bis(2-ethylhexyl)phthalate 37 Bromoform 39 Carbon tetrachloride 42 Chlorobenzene 46 Chloroethane 49 Chloroform 52 Cyclohexane 55 Di-n-butyl phthalate 58 1.1-Dichloroethane 60 1.2-Dichloroethane 63 1,1-Dichloroethene 66 trans-1,2-Dichloroethene 69 Dimethyl sulfoxide 71 1,4-Dioxane 74 Ethanol 77 Ethylbenzene 81 Ethylene dibromide 84 Methanol 87 Methylene chloride 91 Naphthalene 94 Phenol 97 Quinoline 101 Tetrachloroethene 103 Toluene 106 1,1,1-Trichloroethane 109 Trichloroethene 112 Vinyl chloride 115 Water 118 m-Xylene 121 o-Xylene 124 p-Xylene 127 References and Bibliography 130 SUMMARY TABLES page 1. Ranking of top 20 organic ground water contaminants based on number of sites at which each contaminant was detected. 17 2. Selected toxic organic chemicals ordered by Chemical Abstract Service Registry Number (CAS RN). Those chemicals on the U.S. EPA top 100 hazardous substances list are also noted. 18 3. Selected toxic organic chemicals ordered by number of carbon and hydrogen atoms. 19 4. Ranking of selected toxic organic chemicals by specific gravity at room temperature. -

19650003847.Pdf
NATIONAL BUREAU OF STANDARDS REPO RT 8595 PRELIMINARY REPORT ON A SURVEY OF THERMODYNAMIC PROPERTIES OF THE COMPOUNDS OF THE ELEMENTS CHNOPS i N65 2 CACC£S$IO NUMB£:R) CTHRU) ~ ___f£ / I- (PAGES) (CdOEI ::; ~ CIU - d -P?02c2/ :33 (NASA CR OR TMX OR AD NUMBER) (CATEQORY) Progress Report for the Period 1 August to 31 October 1964 to National Aeronautics and Space Administration GPO PRICE $ _____ OTS PRICE(S) $ 1 November 1964 Hard copy (He) of· cJV r Microfiche (MF) __....;... : .:::.j~I?J~_ <@> U.S. DEPARTMENT OF COMMERCE NATIONAL BUREAU OF STANDARDS THE NATIONAL BUREAU OF STANDARD S The ational Bureau of Standards is a principal focal point in the Federal Government for assuring maximum application of the physical and engineering sciences to the advancement of technology in industry and commerce. It responsibilities include development and main tena nce of the national stand· ards of measurement, and the provisions of means for making measurements consi tent with those standard ; determination of physical constants and properties of materials; development of methods for testing materials, mechanisms, and structures, and making such tests as may be nece sary, particu larl: for ~overn ment agencies; cooperation in the establi hment of standard practices for incorpora tion in codes and specifications; advisory service to government agencies on scientific and technical problems; invention and development of device to serve special needs of the Government: assi tance to indus! r). business. and consumers in the development and acceptance of commercial tandard and simplified trade practice recommendations; administration of programs in cooperation with United tates bu iness groups and standards organizations for the development of international standard of practice; and maintenance of a clearinghouse for the collection and dis emination of scientific, tech nical. -

Organic Chemistry of Explosives
JWBK121-FM October 11, 2006 21:10 Char Count= 0 Organic Chemistry of Explosives Dr. Jai Prakash Agrawal CChem FRSC (UK) Former Director of Materials Defence R&D Organisation DRDO House, New Delhi, India email: [email protected] Dr. Robert Dale Hodgson Consultant Organic Chemist, Syntech Chemical Consultancy, Morecambe, Lancashire, UK Website: http://www.syntechconsultancy.co.uk email: [email protected] iii JWBK121-FM October 11, 2006 21:10 Char Count= 0 iii JWBK121-FM October 11, 2006 21:10 Char Count= 0 Organic Chemistry of Explosives i JWBK121-FM October 11, 2006 21:10 Char Count= 0 ii JWBK121-FM October 11, 2006 21:10 Char Count= 0 Organic Chemistry of Explosives Dr. Jai Prakash Agrawal CChem FRSC (UK) Former Director of Materials Defence R&D Organisation DRDO House, New Delhi, India email: [email protected] Dr. Robert Dale Hodgson Consultant Organic Chemist, Syntech Chemical Consultancy, Morecambe, Lancashire, UK Website: http://www.syntechconsultancy.co.uk email: [email protected] iii JWBK121-FM October 11, 2006 21:10 Char Count= 0 Copyright C 2007 John Wiley & Sons Ltd, The Atrium, Southern Gate, Chichester, West Sussex PO19 8SQ, England Telephone (+44) 1243 779777 Email (for orders and customer service enquiries): [email protected] Visit our Home Page on www.wiley.com All Rights Reserved. No part of this publication may be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, mechanical, photocopying, recording, scanning or otherwise, except under the terms of the Copyright, Designs and Patents Act 1988 or under the terms of a licence issued by the Copyright Licensing Agency Ltd, 90 Tottenham Court Road, London W1T 4LP, UK, without the permission in writing of the Publisher. -

(CDR) by CASRN Or Accession Number
List of Chemicals Reported for the 2012 Chemical Data Reporting (CDR) by CASRN or Accession Number For the 2012 CDR, 7,674 unique chemicals were reported by manufacturers (including importers). Chemicals are listed by CAS Registry Number (for non-confidential chemicals) or by TSCA Accession Number (for chemicals listed on the confidential portion of the TSCA Inventory). CASRN or CASRN or ACCESSION ACCESSION NUMBER CA INDEX NAME or GENERIC NAME NUMBER CA INDEX NAME or GENERIC NAME 100016 Benzenamine, 4-nitro- 10042769 Nitric acid, strontium salt (2:1) 10006287 Silicic acid (H2SiO3), potassium salt (1:2) 10043013 Sulfuric acid, aluminum salt (3:2) 1000824 Urea, N-(hydroxymethyl)- 10043115 Boron nitride (BN) 100107 Benzaldehyde, 4-(dimethylamino)- 10043353 Boric acid (H3BO3) 1001354728 4-Octanol, 3-amino- 10043524 Calcium chloride (CaCl2) 100174 Benzene, 1-methoxy-4-nitro- 100436 Pyridine, 4-ethenyl- 10017568 Ethanol, 2,2',2''-nitrilotris-, phosphate (1:?) 10043842 Phosphinic acid, manganese(2+) salt (2:1) 2,7-Anthracenedisulfonic acid, 9,10-dihydro- 100447 Benzene, (chloromethyl)- 10017591 9,10-dioxo-, sodium salt (1:?) 10045951 Nitric acid, neodymium(3+) salt (3:1) 100185 Benzene, 1,4-bis(1-methylethyl)- 100469 Benzenemethanamine 100209 1,4-Benzenedicarbonyl dichloride 100470 Benzonitrile 100210 1,4-Benzenedicarboxylic acid 100481 4-Pyridinecarbonitrile 10022318 Nitric acid, barium salt (2:1) 10048983 Phosphoric acid, barium salt (1:1) 9-Octadecenoic acid (9Z)-, 2-methylpropyl 10049044 Chlorine oxide (ClO2) 10024472 ester Phosphoric acid, -

Table 4: Protective Action Criteria (PAC) Rev. 29 Based on Applicable
Table 4: Protective Action Criteria (PAC) Rev. 29a based on applicable 60-minute AEGLs, ERPGs, or TEELs. The chemicals are 3 listed in alphabetical order and the values are presented in mg/m . June 2018 Table 4 is an alphabetical list of the chemical substances and their corresponding PAC values in mass per unit volume (mg/m3). The conversion of ppm to mg/m3 was carried out assuming normal temperature and pressure, 25°C and 760 mm Hg. The columns presented in Table 4 provide the following information: Heading Definition No. The ordered numbering of the chemicals as they appear in this alphabetical listing Chemical Name The name of the chemical substance submitted to the PAC development team CASRN The Chemical Abstracts Service Registry Number1 for this chemical PAC-1 Based on the applicable AEGL-1, ERPG-1, or TEEL-1 value PAC-2 Based on the applicable AEGL-2, ERPG-2, or TEEL-2 value PAC-3 Based on the applicable AEGL-3, ERPG-3, or TEEL-3 value Chemicals for which AEGLs are available have their chemical name, CASRN, and AEGL values displayed in a bolded and larger font. Chemicals for which ERPGs are available, but not AEGLs, have their chemical name, CASRN, and ERPG values displayed in a bolded font. Chemicals for which TEELs are available, but no AEGLs or ERPGs, have their chemical name, CASRN, and values displayed using a regular font. Additional information on PAC values and TEEL values and links to other sources of information is provided on the Subcommittee on Consequence Assessment and Protective Actions (SCAPA) webpage at http://orise.orau.gov/emi/scapa/default.htm. -

Comparison of the Mms Cn-Dtm Cyanide Destruction Process with Caro's Acid, Smbs/Air and Hydrogen Peroxide Options
COMPARISON OF THE MMS CN-DTM CYANIDE DESTRUCTION PROCESS WITH CARO’S ACID, SMBS/AIR AND HYDROGEN PEROXIDE OPTIONS By 1 Mike Adams 1 Maelgwyn Australia Pty Ltd, PO Box 374, Guildford, WA 6935, Australia Presenter and Corresponding Author Mike Adams [email protected] ABSTRACT Modern gold plants are under increasing pressure to adopt world-class cyanide management prac- tices due to escalating environmental pressures, particularly for signatories to the International Cyanide Management Code. Operations are lowering the levels of weak-acid dissociable (WAD) cyanide reporting to spigot discharge and to any eventual discharges from the tailings storage facili- ty (TSF). Gold operations are increasingly adopting the inclusion of cyanide oxidation processes into their circuits, particularly for greenfields projects. These processes predominantly use sodium meta-bisulfite (SMBS, or SO2) with air, Caro’s acid (H2SO5), or hydrogen peroxide (H2O2). Maelgwyn Mineral Services (MMS) has developed the MMS CN-DTM process, which utilizes the Aa- chen ReactorTM, a high-energy mass-transfer superoxygenation system, in conjunction with an acti- vated carbon-based catalyst, to increase the rate of cyanide oxidation to cyanate, the thermody- namically more stable form. These four processes are compared with respect to stoichiometric rea- gent costs, chemical reaction efficiencies and risks, as well as logistical, operability, safety and envi- ronmental issues, for both remote sites and those close to urban areas. Considerable potential up- side benefit is demonstrated for MMS CN-DTM compared with the alternative cyanide destruction processes. Additional potential features of the MMS CN-DTM process are also considered, such as the potential to recover additional gold otherwise lost to tailings, to de-risk implementation and lower TM capital costs via staged integration of Aachen Reactors into existing oxygenation and SO2/Air equipment and to modify existing plant tankage in CIL trains to become part of the MMS CN-DTM cyanide destruction plant component.