Clinico-Radiological Phenotyping and Diagnostic Pathways in Childhood Neurometabolic Disorders—A Practical Introductory Guide
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hyperammonemia in Review: Pathophysiology, Diagnosis, and Treatment
Pediatr Nephrol DOI 10.1007/s00467-011-1838-5 EDUCATIONAL REVIEW Hyperammonemia in review: pathophysiology, diagnosis, and treatment Ari Auron & Patrick D. Brophy Received: 23 September 2010 /Revised: 9 January 2011 /Accepted: 12 January 2011 # IPNA 2011 Abstract Ammonia is an important source of nitrogen and is the breakdown and catabolism of dietary and bodily proteins, required for amino acid synthesis. It is also necessary for respectively. In healthy individuals, amino acids that are not normal acid-base balance. When present in high concentra- needed for protein synthesis are metabolized in various tions, ammonia is toxic. Endogenous ammonia intoxication chemical pathways, with the rest of the nitrogen waste being can occur when there is impaired capacity of the body to converted to urea. Ammonia is important for normal animal excrete nitrogenous waste, as seen with congenital enzymatic acid-base balance. During exercise, ammonia is produced in deficiencies. A variety of environmental causes and medica- skeletal muscle from deamination of adenosine monophos- tions may also lead to ammonia toxicity. Hyperammonemia phate and amino acid catabolism. In the brain, the latter refers to a clinical condition associated with elevated processes plus the activity of glutamate dehydrogenase ammonia levels manifested by a variety of symptoms and mediate ammonia production. After formation of ammonium signs, including significant central nervous system (CNS) from glutamine, α-ketoglutarate, a byproduct, may be abnormalities. Appropriate and timely management requires a degraded to produce two molecules of bicarbonate, which solid understanding of the fundamental pathophysiology, are then available to buffer acids produced by dietary sources. differential diagnosis, and treatment approaches available. -

Effect of Propionic Acid on Fatty Acid Oxidation and U Reagenesis
Pediat. Res. 10: 683- 686 (1976) Fatty degeneration propionic acid hyperammonemia propionic acidemia liver ureagenesls Effect of Propionic Acid on Fatty Acid Oxidation and U reagenesis ALLEN M. GLASGOW(23) AND H. PET ER C HASE UniversilY of Colorado Medical Celller, B. F. SlOlillsky LaboralOries , Denver, Colorado, USA Extract phosphate-buffered salin e, harvested with a brief treatment wi th tryps in- EDTA, washed twice with ph os ph ate-buffered saline, and Propionic acid significantly inhibited "CO z production from then suspended in ph os ph ate-buffe red saline (145 m M N a, 4.15 [I-"ejpalmitate at a concentration of 10 11 M in control fibroblasts m M K, 140 m M c/, 9.36 m M PO" pH 7.4) . I n mos t cases the cells and 100 11M in methyl malonic fibroblasts. This inhibition was we re incubated in 3 ml phosph ate-bu ffered sa lin e cont aining 0.5 similar to that produced by 4-pentenoic acid. Methylmalonic acid I1Ci ll-I4Cj palm it ate (19), final concentration approximately 3 11M also inhibited ' 'C0 2 production from [V 'ejpalmitate, but only at a added in 10 II I hexane. Increasing the amount of hexane to 100 II I concentration of I mM in control cells and 5 mM in methyl malonic did not impair palmit ate ox id ation. In two experiments (Fig. 3) the cells. fibroblasts were in cub ated in 3 ml calcium-free Krebs-Ringer Propionic acid (5 mM) also inhibited ureagenesis in rat liver phosphate buffer (2) co nt ain in g 5 g/ 100 ml essent iall y fatty ac id slices when ammonia was the substrate but not with aspartate and free bovine se rum albumin (20), I mM pa lm itate, and the same citrulline as substrates. -
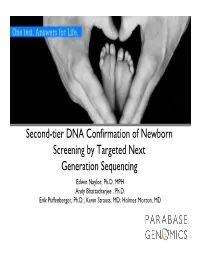
Second-Tier DNA Confirmation of Newborn Screening Results
Second-tier DNA Confirmation of Newborn Screening by Targeted Next Generation Sequencing Edwin Naylor, Ph.D. MPH Andy Bhattacharjee , Ph.D. Erik Puffenberger, Ph.D.; Kevin Strauss, MD; Holmes Morton, MD Newborn Screening & Clinical Genomics 1961 1990’s 2010-2012 2 Robert Guthrie Development of develops simple automated MS/MS Newborn Screening screening across (NBS) several disorders Current de facto standard 2 Why Newborn Genomics? • Mendelian Diseases disproportionately affect Newborns - ~3500 genetic diseases with molecular basis - >10% of NICU admissions are genetic Clinical manifestation of Genetic diseases - Current NBS tests limited to 29+ diseases CHROMOSOMAL - 2nd tier DNA testing to validate biochemical results MULTI-FACTORIAL SINGLE GENE (MENDELIAN) • Advantage of NGS based DNA testing individuals # of Affected - Find causal variants (rare/novel) in gene(s) - A ‘universal’ NGS approach avoids repeated, serial BIRTH PUBERTY ADULT single gene testing Gelehrter TD, Collins FS, Ginsburg D. Principles of - Current Sanger sequencing is expensive ($3-10K) and Medical Genetics. 2nd ed. Baltimore, MD: Williams & slow (3 months to 1 year) Wilkins; 1998:1-42 NICU- Neonatal Intensive Care Unit NBS-Newborn Screening 3 NGS-Next Generation Sequencing Why Targeted (Exome) Sequencing for now? NGS Sequencing * Genomic DNA from Causal Mutations in Affected Individuals Exons/Target Regions Fold Test Menu Cost ($) Throughput Efficiency Whole Genome (Res.) 7,666* 1 1 Exome (Res) 1,200 15 95 Neonate Panel (Clinical) <1000 150 >1140 •Majority of known disease-causing mutations in exons •Exome = protein-encoding parts of genes •Targeted NGS is Cost & Throughput Efficient *Saunders et al., (2012) Rapid Whole Genome Sequencing for Genetic Disease Diagnosis in NICUs 4 Workflow for 2nd Tier Newborn Screening Sample 2h DNA Capture 92h Raw Data 10h Analysis 1h+ Isolation & Sequencing Management & Interpretation 8 samples, 105 Hrs, <$10,000 = Real Neonatal Genomics! 5 Workflow for 2nd Tier Newborn Screening Sample •High M.Wt. -

Newborn Screening Laboratory Manual of Services
Newborn Screening Laboratory Manual of Services Test Panel: Please see the following links for a detailed description of testing in the Newborn Screening section. Information about the Newborn Screening program is available here. Endocrine Disorders Congenital adrenal hyperplasia (CAH) Congenital hypothyroidism (TSH) Hemoglobinopathies Sickle cell disease (FS) Alpha (Barts) Sickle βeta Thalassemia (FSA) Other sickling hemoglobinopathies such as: FAS FAC FAD FAE Homozygous conditions such as: FC FD FE Metabolic Disorders Biotinidase deficiency Galactosemia Cystic fibrosis (CF) first tier screening for elevated immunoreactive trypsinogen (IRT) Cystic fibrosis second tier genetic mutation analysis on the top 4% IRT concentrations. Current alleles detected : F508del, I507del, G542X, G85E, R117H, 621+1G->T, 711+1G->T, R334W, R347P, A455E, 1717-1G->A, R560T, R553X, G551D, 1898+1G->A, 2184delA, 2789+5G->A, 3120+1G->A, R1162X, 3659delC, 3849+10kbC->T, W1282X, N1303K, IVS polyT T5/T7/T9 *Currently validating a mutation panel that includes the above alleles in addition to the following: 1078delT, Y122X, 394delTT, R347H, M1101K, S1255X, 1898+5G->T, 2183AA->G, 2307insA, Y1092X, 3876delA, 3905insT, S549N, S549R_1645A->C, S549R-1647T->G, S549R-1647T->G, V520F, A559T, 1677delTA, 2055del9->A, 2143delT, 3199del6, 406-1G->A, 935delA, D1152H, CFTRdele2, E60X, G178R, G330X, K710X, L206W, Q493X, Q890X, R1066C, R1158X, R75X, S1196X, W1089X, G1244E, G1349D, G551S, R560KT, S1251N, S1255P Amino acid disorders Phenylketonuria (PKU) / Hyperphenylalaninemia Maple -

CBS, MET Act Sheet
Newborn Screening ACT Sheet Increased Methionine Homocystinuria (CBS Deficiency) / Hypermethioninemia (MET) Differential Diagnosis: Classical homocystinuria (cystathionine β-synthase (CBS) deficiency); hypermethioninemia (MET) due to methionine adenosyltransferase I/III MAT I/III deficiency; glycine n-methyltransferase GNMT deficiency; adenosylhomocysteine hydrolase deficiency; liver disease; hyperalimentation. Condition Description: Methionine from ingested protein is normally converted to homocysteine. In classical homocystinuria due to CBS deficiency, homocysteine cannot be converted to cystathionine. As a result, the concentration of homocysteine and its precursor, methionine, will become elevated. In MAT I/III deficiency and the other hypermethioninemias, methionine is increased in the absence of, or only with, a slightly increased level of homocysteine. You Should Take the Following IMMEDIATE Actions • Contact family to inform them of the newborn screening result and ascertain clinical status. • Consult with pediatric metabolic specialist. (See attached list.) • Evaluate the newborn with attention to liver disease and refer as appropriate. • Initiate confirmatory/diagnostic tests in consultation with metabolic specialist. • Initial testing: Plasma quantitative amino acids and plasma total homocysteine. • Repeat newborn screen if second screen has not been done. • Educate family about homocystinuria and its management as appropriate. • Report findings to newborn screening program. Diagnostic Evaluation: Plasma quantitative amino -

Disorders Included in the Newborn Screening Panel Disorders
Disorders Included in the Newborn Screening Panel Disorders Detected by Tandem Mass Spectrometry Acylcarnitine Profile Amino Acid Profile Fatty Acid Oxidation Disorders Amino Acid Disorders Carnitine/Acylcarnitine Translocase Deficiency Argininemia 1 Carnitine Palmitoyl Transferase Deficiency Type I Argininosuccinic Aciduria 1 3-Hydroxy Long Chain Acyl-CoA Dehydrogenase 5-Oxoprolinuria 1 Deficiency Carbamoylphosphate Synthetase Deficiency 1 2,4-Dienoyl-CoA Reductase Deficiency Citrullinemia Medium Chain Acyl-CoA Dehydrogenase Deficiency Homocystinuria Multiple Acyl-CoA Dehydrogenase Deficiency Hypermethioninemia Neonatal Carnitine Palmitoyl Transferase Deficiency Hyperammonemia, Hyperornithinemia, Homocitrullinuria Type II Syndrome1 1 Short Chain Acyl-CoA Dehydrogenase Deficiency Hyperornithinemia with Gyral Atrophy Short Chain Hydroxy Acyl-CoA Dehydrogenase Maple Syrup Urine Disease Deficiency Phenylketonuria Trifunctional Protein Deficiency Classical/Hyperphenylalaninemia Very Long Chain Acyl-CoA Dehydrogenase Deficiency Biopterin Cofactor Deficiencies Tyrosinemia Organic Acid Disorders Transient Neonatal Tyrosinemia 2 Tyrosinemia Type I 3-Hydroxy-3-Methylglutaryl-CoA Lyase Deficiency Tyrosinemia Type II Glutaric Acidemia Type I Tyrosinemia Type III Isobutyryl-CoA Dehydrogenase Deficiency Isovaleric Acidemia 2-Methylbutyryl-CoA Dehydrogenase Deficiency 3-Methylcrotonyl-CoA Carboxylase Deficiency Other Observations 3-Methylglutaconyl-CoA Hydratase Deficiency Methylmalonic Acidemias Hyperalimentation Methylmalonyl-CoA Mutase Deficiency -

Incidence of Inborn Errors of Metabolism by Expanded Newborn
Original Article Journal of Inborn Errors of Metabolism & Screening 2016, Volume 4: 1–8 Incidence of Inborn Errors of Metabolism ª The Author(s) 2016 DOI: 10.1177/2326409816669027 by Expanded Newborn Screening iem.sagepub.com in a Mexican Hospital Consuelo Cantu´-Reyna, MD1,2, Luis Manuel Zepeda, MD1,2, Rene´ Montemayor, MD3, Santiago Benavides, MD3, Hector´ Javier Gonza´lez, MD3, Mercedes Va´zquez-Cantu´,BS1,4, and Hector´ Cruz-Camino, BS1,5 Abstract Newborn screening for the detection of inborn errors of metabolism (IEM), endocrinopathies, hemoglobinopathies, and other disorders is a public health initiative aimed at identifying specific diseases in a timely manner. Mexico initiated newborn screening in 1973, but the national incidence of this group of diseases is unknown or uncertain due to the lack of large sample sizes of expanded newborn screening (ENS) programs and lack of related publications. The incidence of a specific group of IEM, endocrinopathies, hemoglobinopathies, and other disorders in newborns was obtained from a Mexican hospital. These newborns were part of a comprehensive ENS program at Ginequito (a private hospital in Mexico), from January 2012 to August 2014. The retrospective study included the examination of 10 000 newborns’ results obtained from the ENS program (comprising the possible detection of more than 50 screened disorders). The findings were the following: 34 newborns were confirmed with an IEM, endocrinopathies, hemoglobinopathies, or other disorders and 68 were identified as carriers. Consequently, the estimated global incidence for those disorders was 3.4 in 1000 newborns; and the carrier prevalence was 6.8 in 1000. Moreover, a 0.04% false-positive rate was unveiled as soon as diagnostic testing revealed negative results. -

Mutation of the Fumarase Gene in Two Siblings with Progressive Encephalopathy and Fumarase Deficiency T
Mutation of the Fumarase Gene in Two Siblings with Progressive Encephalopathy and Fumarase Deficiency T. Bourgeron,* D. Chretien,* J. Poggi-Bach, S. Doonan,' D. Rabier,* P. Letouze,I A. Munnich,* A. R6tig,* P. Landneu,* and P. Rustin* *Unite de Recherches sur les Handicaps Genetiques de l'Enfant, INSERM U393, Departement de Pediatrie et Departement de Biochimie, H6pital des Enfants-Malades, 149, rue de Sevres, 75743 Paris Cedex 15, France; tDepartement de Pediatrie, Service de Neurologie et Laboratoire de Biochimie, Hopital du Kremlin-Bicetre, France; IFaculty ofScience, University ofEast-London, UK; and IService de Pediatrie, Hopital de Dreux, France Abstract chondrial enzyme (7). Human tissue fumarase is almost We report an inborn error of the tricarboxylic acid cycle, fu- equally distributed between the mitochondria, where the en- marase deficiency, in two siblings born to first cousin parents. zyme catalyzes the reversible hydration of fumarate to malate They presented with progressive encephalopathy, dystonia, as a part ofthe tricarboxylic acid cycle, and the cytosol, where it leucopenia, and neutropenia. Elevation oflactate in the cerebro- is involved in the metabolism of the fumarate released by the spinal fluid and high fumarate excretion in the urine led us to urea cycle. The two isoenzymes have quite homologous struc- investigate the activities of the respiratory chain and of the tures. In rat liver, they differ only by the acetylation of the Krebs cycle, and to finally identify fumarase deficiency in these NH2-terminal amino acid of the cytosolic form (8). In all spe- two children. The deficiency was profound and present in all cies investigated so far, the two isoenzymes have been found to tissues investigated, affecting the cytosolic and the mitochon- be encoded by a single gene (9,10). -

Inherited Metabolic Disease
Inherited metabolic disease Dr Neil W Hopper SRH Areas for discussion • Introduction to IEMs • Presentation • Initial treatment and investigation of IEMs • Hypoglycaemia • Hyperammonaemia • Other presentations • Management of intercurrent illness • Chronic management Inherited Metabolic Diseases • Result from a block to an essential pathway in the body's metabolism. • Huge number of conditions • All rare – very rare (except for one – 1:500) • Presentation can be non-specific so index of suspicion important • Mostly AR inheritance – ask about consanguinity Incidence (W. Midlands) • Amino acid disorders (excluding phenylketonuria) — 18.7 per 100,000 • Phenylketonuria — 8.1 per 100,000 • Organic acidemias — 12.6 per 100,000 • Urea cycle diseases — 4.5 per 100,000 • Glycogen storage diseases — 6.8 per 100,000 • Lysosomal storage diseases — 19.3 per 100,000 • Peroxisomal disorders — 7.4 per 100,000 • Mitochondrial diseases — 20.3 per 100,000 Pathophysiological classification • Disorders that result in toxic accumulation – Disorders of protein metabolism (eg, amino acidopathies, organic acidopathies, urea cycle defects) – Disorders of carbohydrate intolerance – Lysosomal storage disorders • Disorders of energy production, utilization – Fatty acid oxidation defects – Disorders of carbohydrate utilization, production (ie, glycogen storage disorders, disorders of gluconeogenesis and glycogenolysis) – Mitochondrial disorders – Peroxisomal disorders IMD presentations • ? IMD presentations • Screening – MCAD, PKU • Progressive unexplained neonatal -

Glutaric Acidemia Type 1
Glutaric acidemia type 1 What is glutaric acidemia type 1? Glutaric acidemia type 1 is an inherited disease characterized by episodes of severe brain dysfunction that result in spasticity, low muscle tone, and seizures.1,2 Individuals with glutaric acidemia type 1 have defects in the glutaryl-CoA dehydrogenase enzyme, which breaks down the amino acids lysine, hydroxylysine, and tryptophan. The symptoms of glutaric acidemia type 1 are due to the build-up of these amino acids and their metabolites in the body, primarily affecting the brain.1 Glutaric acidemia type 1 is also known as glutaric aciduria type 1.2 What are the symptoms of glutaric acidemia type 1 and what treatment is available? The severity of symptoms of glutaric acidemia type 1 can vary widely, even within families. Newborns may have macrocephaly (large head size) with no other signs or symptoms. Symptoms typically begin within months after birth and are often triggered by illness or fasting. Symptoms may include2: • Hypotonia (low muscle tone) • Feeding difficulties • Poor growth • Swelling of the brain • Spasticity (abnormally tight muscles) • Dystonia (sustained muscle contractions causing twisting movements and abnormal posture) • Seizures • Developmental delays • Coma, and possibly death, especially if untreated Individuals tend to have a reduced life expectancy. Approximately 10% of individuals die within the first decade; more than half do not survive past 35 years of age. 3 There is no cure for glutaric acidemia type 1, and treatment is aimed at preventing episodes of brain dysfunction and seizures. Treatment generally includes a low protein diet and nutrition supplements, and a feeding tube may be required for some individuals. -
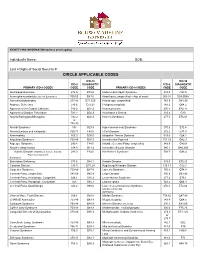
Circle Applicable Codes
IDENTIFYING INFORMATION (please print legibly) Individual’s Name: DOB: Last 4 Digits of Social Security #: CIRCLE APPLICABLE CODES ICD-10 ICD-10 ICD-9 DIAGNOSTIC ICD-9 DIAGNOSTIC PRIMARY ICD-9 CODES CODE CODE PRIMARY ICD-9 CODES CODE CODE Abetalipoproteinemia 272.5 E78.6 Hallervorden-Spatz Syndrome 333.0 G23.0 Acrocephalosyndactyly (Apert’s Syndrome) 755.55 Q87.0 Head Injury, unspecified – Age of onset: 959.01 S09.90XA Adrenaleukodystrophy 277.86 E71.529 Hemiplegia, unspecified 342.9 G81.90 Arginase Deficiency 270.6 E72.21 Holoprosencephaly 742.2 Q04.2 Agenesis of the Corpus Callosum 742.2 Q04.3 Homocystinuria 270.4 E72.11 Agenesis of Septum Pellucidum 742.2 Q04.3 Huntington’s Chorea 333.4 G10 Argyria/Pachygyria/Microgyria 742.2 Q04.3 Hurler’s Syndrome 277.5 E76.01 or 758.33 Aicardi Syndrome 333 G23.8 Hyperammonemia Syndrome 270.6 E72.4 Alcohol Embryo and Fetopathy 760.71 F84.5 I-Cell Disease 272.2 E77.0 Anencephaly 655.0 Q00.0 Idiopathic Torsion Dystonia 333.6 G24.1 Angelman Syndrome 759.89 Q93.5 Incontinentia Pigmenti 757.33 Q82.3 Asperger Syndrome 299.8 F84.5 Infantile Cerebral Palsy, unspecified 343.9 G80.9 Ataxia-Telangiectasia 334.8 G11.3 Intractable Seizure Disorder 345.1 G40.309 Autistic Disorder (Childhood Autism, Infantile 299.0 F84.0 Klinefelter’s Syndrome 758.7 Q98.4 Psychosis, Kanner’s Syndrome) Biotinidase Deficiency 277.6 D84.1 Krabbe Disease 333.0 E75.23 Canavan Disease 330.0 E75.29 Kugelberg-Welander Disease 335.11 G12.1 Carpenter Syndrome 759.89 Q87.0 Larsen’s Syndrome 755.8 Q74.8 Cerebral Palsy, unspecified 343.69 G80.9 -

2018 Etiologies by Frequencies
2018 Etiologies in Order of Frequency by Category Hereditary Syndromes and Disorders Count CHARGE Syndrome 958 Down syndrome (Trisomy 21 syndrome) 308 Usher I syndrome 252 Stickler syndrome 130 Dandy Walker syndrome 119 Cornelia de Lange 102 Goldenhar syndrome 98 Usher II syndrome 83 Wolf-Hirschhorn syndrome (Trisomy 4p) 68 Trisomy 13 (Trisomy 13-15, Patau syndrome) 60 Pierre-Robin syndrome 57 Moebius syndrome 55 Trisomy 18 (Edwards syndrome) 52 Norrie disease 38 Leber congenital amaurosis 35 Chromosome 18, Ring 18 31 Aicardi syndrome 29 Alstrom syndrome 27 Pfieffer syndrome 27 Treacher Collins syndrome 27 Waardenburg syndrome 27 Marshall syndrome 25 Refsum syndrome 21 Cri du chat syndrome (Chromosome 5p- synd) 16 Bardet-Biedl syndrome (Laurence Moon-Biedl) 15 Hurler syndrome (MPS I-H) 15 Crouzon syndrome (Craniofacial Dysotosis) 13 NF1 - Neurofibromatosis (von Recklinghausen dis) 13 Kniest Dysplasia 12 Turner syndrome 11 Usher III syndrome 10 Cockayne syndrome 9 Apert syndrome/Acrocephalosyndactyly, Type 1 8 Leigh Disease 8 Alport syndrome 6 Monosomy 10p 6 NF2 - Bilateral Acoustic Neurofibromatosis 6 Batten disease 5 Kearns-Sayre syndrome 5 Klippel-Feil sequence 5 Hereditary Syndromes and Disorders Count Prader-Willi 5 Sturge-Weber syndrome 5 Marfan syndrome 3 Hand-Schuller-Christian (Histiocytosis X) 2 Hunter Syndrome (MPS II) 2 Maroteaux-Lamy syndrome (MPS VI) 2 Morquio syndrome (MPS IV-B) 2 Optico-Cochleo-Dentate Degeneration 2 Smith-Lemli-Opitz (SLO) syndrome 2 Wildervanck syndrome 2 Herpes-Zoster (or Hunt) 1 Vogt-Koyanagi-Harada