Cellular Iron Uptake, Trafficking and Metabolism
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Supplementary Materials
1 Supplementary Materials: Supplemental Figure 1. Gene expression profiles of kidneys in the Fcgr2b-/- and Fcgr2b-/-. Stinggt/gt mice. (A) A heat map of microarray data show the genes that significantly changed up to 2 fold compared between Fcgr2b-/- and Fcgr2b-/-. Stinggt/gt mice (N=4 mice per group; p<0.05). Data show in log2 (sample/wild-type). 2 Supplemental Figure 2. Sting signaling is essential for immuno-phenotypes of the Fcgr2b-/-lupus mice. (A-C) Flow cytometry analysis of splenocytes isolated from wild-type, Fcgr2b-/- and Fcgr2b-/-. Stinggt/gt mice at the age of 6-7 months (N= 13-14 per group). Data shown in the percentage of (A) CD4+ ICOS+ cells, (B) B220+ I-Ab+ cells and (C) CD138+ cells. Data show as mean ± SEM (*p < 0.05, **p<0.01 and ***p<0.001). 3 Supplemental Figure 3. Phenotypes of Sting activated dendritic cells. (A) Representative of western blot analysis from immunoprecipitation with Sting of Fcgr2b-/- mice (N= 4). The band was shown in STING protein of activated BMDC with DMXAA at 0, 3 and 6 hr. and phosphorylation of STING at Ser357. (B) Mass spectra of phosphorylation of STING at Ser357 of activated BMDC from Fcgr2b-/- mice after stimulated with DMXAA for 3 hour and followed by immunoprecipitation with STING. (C) Sting-activated BMDC were co-cultured with LYN inhibitor PP2 and analyzed by flow cytometry, which showed the mean fluorescence intensity (MFI) of IAb expressing DC (N = 3 mice per group). 4 Supplemental Table 1. Lists of up and down of regulated proteins Accession No. -

Noncanonical Coproporphyrin-Dependent Bacterial Heme Biosynthesis Pathway That Does Not Use Protoporphyrin
Noncanonical coproporphyrin-dependent bacterial heme biosynthesis pathway that does not use protoporphyrin Harry A. Daileya,b,c,1, Svetlana Gerdesd, Tamara A. Daileya,b,c, Joseph S. Burcha, and John D. Phillipse aBiomedical and Health Sciences Institute and Departments of bMicrobiology and cBiochemistry and Molecular Biology, University of Georgia, Athens, GA 30602; dMathematics and Computer Science Division, Argonne National Laboratory, Argonne, IL 60439; and eDivision of Hematology, Department of Medicine, University of Utah School of Medicine, Salt Lake City, UT 84132 Edited by J. Clark Lagarias, University of California, Davis, CA, and approved January 12, 2015 (received for review August 25, 2014) It has been generally accepted that biosynthesis of protoheme of a “primitive” pathway in Desulfovibrio vulgaris (13). This path- (heme) uses a common set of core metabolic intermediates that way, named the “alternative heme biosynthesis” path (or ahb), has includes protoporphyrin. Herein, we show that the Actinobacteria now been characterized by Warren and coworkers (15) in sulfate- and Firmicutes (high-GC and low-GC Gram-positive bacteria) are reducing bacteria. In the ahb pathway, siroheme, synthesized unable to synthesize protoporphyrin. Instead, they oxidize copro- from uroporphyrinogen III, can be further metabolized by suc- porphyrinogen to coproporphyrin, insert ferrous iron to make Fe- cessive demethylation and decarboxylation to yield protoheme (14, coproporphyrin (coproheme), and then decarboxylate coproheme 15) (Fig. 1 and Fig. S1). A similar pathway exists for protoheme- to generate protoheme. This pathway is specified by three genes containing archaea (15, 16). named hemY, hemH, and hemQ. The analysis of 982 representa- Current gene annotations suggest that all enzymes for pro- tive prokaryotic genomes is consistent with this pathway being karyotic heme synthetic pathways are now identified. -

The Ferritin Fe2 Site at the Diiron Catalytic Center Controls the Reaction with O2 in the Rapid Mineralization Pathway
The ferritin Fe2 site at the diiron catalytic center controls the reaction with O2 in the rapid mineralization pathway Takehiko Toshaa, Mohammad R. Hasana,1, and Elizabeth C. Theila,b,2 aCouncil on BioIron at Children’s Hospital Oakland Research Institute, 5700 Martin Luther King, Jr., Way, Oakland, CA 94609; and bDepartment of Nutritional Sciences and Toxicology, University of California, Berkeley, CA 94720 Edited by Edward I. Solomon, Stanford University, Stanford, CA, and approved October 13, 2008 (received for review June 2, 2008) Oxidoreduction in ferritin protein nanocages occurs at sites that Oxidoreductase/ferroxidase (Fox) catalytic centers with difer- bind two Fe(II) substrate ions and O2, releasing Fe(III)2–O products, rous binding sites, Fe1 and Fe2, occur in each ferritin subunit the biomineral precursors. Diferric peroxo intermediates form in except in animals, where a second gene encodes a catalytically ferritins and in the related diiron cofactor oxygenases. Cofactor inactive subunit (ferritin L) that co-assembles with the active iron is retained at diiron sites throughout catalysis, contrasting subunits in various ratios that depend on the tissue. The ferritin with ferritin. Four of the 6 active site residues are the same in oxidoreductase sites share properties with diiron cofactor cata- ferritins and diiron oxygenases; ferritin-specific Gln137 and variable lysts that include the first detectable reaction intermediate in Asp/Ser/Ala140 substitute for Glu and His, respectively, in diiron ferritin, a diferric peroxo (DFP) complex, characterized by cofactor active sites. To understand the selective functions of UV/visible (UV/vis), resonance Raman, Mo¨ssbauer, and ex- diiron substrate and diiron cofactor active site residues, we com- tended X-ray absorption fine structure (EXAFS) spectroscopies pared oxidoreductase activity in ferritin with diiron cofactor resi- (8–14). -

Metabolism of the Stimulated Rat Spleen: I. Ferrochelatase Activity As an Index of Tissue Erythropoiesis
Metabolism of the stimulated rat spleen: I. Ferrochelatase activity as an index of tissue erythropoiesis Abraham Mazur J Clin Invest. 1968;47(10):2230-2238. https://doi.org/10.1172/JCI105908. Assay of the enzyme ferrochelatase in marrow, liver, spleen, and red cells has been employed to assess the extent of erythropoietic stimulation in animals bearing the Walker 256 carcinosarcoma and in rats treated by administration of phenylhydrazine, cobalt chloride, human urinary erythropoietin, or chronic blood loss. In all instances, the spleen sustains the most marked increase of ferrochelatase activity, per gram of tissue. Spleen erythropoietic activity stimulation was confirmed by quantitative measurements in respiring slices of 59Fe and 14C incorporation into hemoglobin and ferritin. Increased spleen ferrochelatase activity in cobalt chloride-treated rats is prevented by actinomycin D, indicating that stimulated synthesis of the enzyme is associated with the metabolism of RNA. Find the latest version: https://jci.me/105908/pdf Metabolism of the Stimulated Rat Spleen I. FERROCHELATASE ACTIVITY AS AN INDEX OF TISSUE ERYTHROPOIESIS ABRAHAM MAZUR From The New York Blood Center, New York 10021 A B S TR A C T Assay of the enzyme ferrochelatase examination or the measurement of incorporation in marrow, liver, spleen, and red cells has been of injected 59Fe into the tissues (4). In addition, employed to assess the extent of erythropoietic other splenic cells (reticuloendothelial cells) may stimulation in animals bearing the Walker 256 car- hypertrophy, e.g., in response to phenylhydrazine cinosarcoma and in rats treated by administration administration (5). of phenylhydrazine, cobalt chloride, human urinary Because the entire spleen is readily available, erythropoietin, or chronic blood loss. -

Supplementary Materials
Supplementary Materials COMPARATIVE ANALYSIS OF THE TRANSCRIPTOME, PROTEOME AND miRNA PROFILE OF KUPFFER CELLS AND MONOCYTES Andrey Elchaninov1,3*, Anastasiya Lokhonina1,3, Maria Nikitina2, Polina Vishnyakova1,3, Andrey Makarov1, Irina Arutyunyan1, Anastasiya Poltavets1, Evgeniya Kananykhina2, Sergey Kovalchuk4, Evgeny Karpulevich5,6, Galina Bolshakova2, Gennady Sukhikh1, Timur Fatkhudinov2,3 1 Laboratory of Regenerative Medicine, National Medical Research Center for Obstetrics, Gynecology and Perinatology Named after Academician V.I. Kulakov of Ministry of Healthcare of Russian Federation, Moscow, Russia 2 Laboratory of Growth and Development, Scientific Research Institute of Human Morphology, Moscow, Russia 3 Histology Department, Medical Institute, Peoples' Friendship University of Russia, Moscow, Russia 4 Laboratory of Bioinformatic methods for Combinatorial Chemistry and Biology, Shemyakin-Ovchinnikov Institute of Bioorganic Chemistry of the Russian Academy of Sciences, Moscow, Russia 5 Information Systems Department, Ivannikov Institute for System Programming of the Russian Academy of Sciences, Moscow, Russia 6 Genome Engineering Laboratory, Moscow Institute of Physics and Technology, Dolgoprudny, Moscow Region, Russia Figure S1. Flow cytometry analysis of unsorted blood sample. Representative forward, side scattering and histogram are shown. The proportions of negative cells were determined in relation to the isotype controls. The percentages of positive cells are indicated. The blue curve corresponds to the isotype control. Figure S2. Flow cytometry analysis of unsorted liver stromal cells. Representative forward, side scattering and histogram are shown. The proportions of negative cells were determined in relation to the isotype controls. The percentages of positive cells are indicated. The blue curve corresponds to the isotype control. Figure S3. MiRNAs expression analysis in monocytes and Kupffer cells. Full-length of heatmaps are presented. -
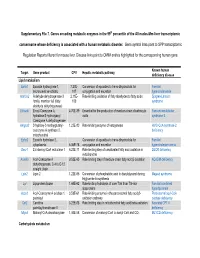
Supplementary File 7. Genes Encoding Metabolic Enzymes in The
Supplementary File 7. Genes encoding metabolic enzymes in the 99th percentile of the All nodes-Mm-liver transcriptomic consensome whose deficiency is associated with a human metabolic disorder. Gene symbol links point to SPP transcriptomic Regulation Reports filtered for mouse liver. Disease links point to OMIM entries highlighted for the corresponding human gene. Known human Target Gene product CPV Hepatic metabolic pathway deficiency disease Lipid metabolism Ephx1 Epoxide hydroxylase 1, 7.27E- Conversion of epoxides to trans-dihydrodiols for Familial microsomal xenobiotic 107 conjugation and excretion hypercholanemia Aldh3a2 Aldehyde dehydrogenase 3 2.11E- Rate-limiting oxidation of fatty aldehydes to fatty acids Sjogren-Larsson family, member A2 (fatty 103 syndrome aldehyde dehydrogenase) Ehhadh Enoyl-Coenzyme A, 4.70E-89 Essential for the production of medium-chain dicarboxylic Fanconi renotubular hydratase/3-hydroxyacyl acids syndrome 3 Coenzyme A dehydrogenase Hmgcs2 3-hydroxy-3-methylglutaryl- 1.23E-80 Rate-limiting enzyme of ketogenesis HMG-CoA synthase-2 coenzyme A synthase 2, deficiency mitochondrial Ephx2 Epoxide hydrolase 2, Conversion of epoxides to trans-dihydrodiols for Familial cytoplasmic 6.06E-78 conjugation and excretion hypercholesterolemia Decr1 2,4-dienoyl-CoA reductase 1 6.23E-71 Rate-limiting step of unsaturated fatty acid oxidation in DECR deficiency mitochondria Acadm Acyl-Coenzyme A 9.52E-65 Rate limiting step of medium-chain fatty acid β-oxidation ACADM deficiency dehydrogenase, C-4 to C-12 straight chain Lpin2 -

The Construction and Characterization of Mitochondrial Ferritin Overexpressing Mice
Article The Construction and Characterization of Mitochondrial Ferritin Overexpressing Mice Xin Li 1,†, Peina Wang 1,†, Qiong Wu 1, Lide Xie 2, Yanmei Cui 1, Haiyan Li 1, Peng Yu 1 and Yan-Zhong Chang 1,* 1 Laboratory of Molecular Iron Metabolism, The Key Laboratory of Animal Physiology, Biochemistry and Molecular Biology of Hebei Province, College of Life Science, Hebei Normal University, Shijiazhuang 050024, China; [email protected] (X.L.); [email protected] (P.W.); [email protected] (Q.W.); [email protected] (Y.C.); [email protected] (H.L.); [email protected] (P.Y.) 2 Department of Biomedical Engineering, Chengde Medical University, Chengde 067000, China; [email protected] * Correspondence: [email protected]; Tel.: +86-311-8078-6311 † These authors contributed equally to this work. Received: 31 May 2017; Accepted: 10 July 2017; Published: 13 July 2017 Abstract: Mitochondrial ferritin (FtMt) is a H-ferritin-like protein which localizes to mitochondria. Previous studies have shown that this protein can protect mitochondria from iron-induced oxidative damage, while FtMt overexpression in cultured cells decreases cytosolic iron availability and protects against oxidative damage. To investigate the in vivo role of FtMt, we established FtMt overexpressing mice by pro-nucleus microinjection and examined the characteristics of the animals. We first confirmed that the protein levels of FtMt in the transgenic mice were increased compared to wild-type mice. Interestingly, we found no significant differences in the body weights or organ to body weight ratios between wild type and transgenic mice. To determine the effects of FtMt overexpression on baseline murine iron metabolism and hematological indices, we measured serum, heart, liver, spleen, kidney, testis, and brain iron concentrations, liver hepcidin expression and red blood cell parameters. -

Some Biochemical Changes in Heme Synthesis in Iron Deficiency
Indian J Physiol Pharmacol 2000; 44 (4): 491-494 SOME BIOCHEMICAL CHANGES IN HEME SYNTHESIS IN IRON DEFICIENCY D. C. SHARMA* AND RATI MATHUR Department of Biochemistry, S. M. S. Medical College, Jaipur - 302 004 (Received on January 18, 2000) Abstract: Some enzymes and intermediates of heme synthesis were determined in blood and urine of 26 women with severe iron deficiency anemia (IDA). Erythrocyte free protoporphyrin was almost doubled and delta-aminolevulinate dehydrase significantly raised. But urinary excretion of delta-aminolevulinic acid and reticulocyte ferrochelatase were significantly reduced in iron deficiency anemia. Hence these could serve as useful indices of iron deficiency and consequent anemia. Key words: iron deficiency anemia delta-aminolevulinate dehydrase protoporphyrin ferrochelatase delta-aminolevulinic acid INTRODUCTION iron incorporation at the level of ferrochelatase. However, this enzyme was Anemia is the chief manifestation of never measured in blood in IDA, despite iron deficiency. Several parameters are reports of its decrease in human leucocytes available for diagnosis of iron deficiency (3) and pig heart muscle (4). Therefore, we anemia (IDA). These include classical decided to estimate it. parameters, like, erythrocyte morphology, red cell indices, bone marrow iron, Another enzyme of heme biosynthesis- serum iron, iron binding capacity, delta aminolevulinate dehydrase (ALAD) has transferrin saturation, and more recent been studied in IDA in the past but the tests-erythrocyte protoporphyrin, serum reports were conflicting; ranging from ferritin, transferrin receptors (1) and zinc normal (5) to higher (6-8) or reduced (3). protoporphyrin (2). Similar contradiction was also seen in the reported excretion of delta-aminolevulinic Iron is known to affect synthesis of heme acid (ALA) in urine by iron deficient anemic and free protoporphyrin accumulates in persons in this study. -

Complex Hereditary Spastic Paraplegia Associated with Episodic
Tozawa et al. Human Genome Variation (2021) 8:4 https://doi.org/10.1038/s41439-021-00136-y Human Genome Variation DATA REPORT Open Access Complex hereditary spastic paraplegia associated with episodic visual loss caused by ACO2 variants Takenori Tozawa1,2,AkiraNishimura3, Tamaki Ueno2,4, Akane Shikata5, Yoshihiro Taura1,TakeshiYoshida 6, Naoko Nakagawa7, Takahito Wada 7, Shinji Kosugi7, Tomoko Uehara8, Toshiki Takenouchi 9, Kenjiro Kosaki8 and Tomohiro Chiyonobu1 Abstract Most patients with homozygous or compound heterozygous pathogenic ACO2 variants present with muscular hypotonia features, namely, infantile cerebellar-retinal degeneration. Recently, two studies reported rare familial cases of ACO2 variants presenting as complex hereditary spastic paraplegia (HSP) with broad clinical spectra. Here, we report the case of a 20-year-old Japanese woman with complex HSP caused by compound heterozygous ACO2 variants, revealing a new phenotype of episodic visual loss during febrile illness. The ACO2 gene on chromosome 22 encodes the aco- variants in the ACO2 gene presenting as complex her- nitase 2 (ACO2) protein in the mitochondrial matrix; editary spastic paraplegia (HSP) with a new phenotype of ACO2 catalyzes the stereospecific isomerization of citrate episodic visual loss after every febrile infection and pro- to isocitrate in the tricarboxylic acid (TCA) cycle1. gressive optic atrophy. This is the third familial report and ACO2 fi fi 1234567890():,; 1234567890():,; 1234567890():,; 1234567890():,; Pathogenic variants were rst reported in eight the rst Asian patient with complex HSP caused by individuals from two Arab families, and they had infantile pathogenic ACO2 variants. cerebellar-retinal degeneration (ICRD, OMIM#614559)2. The proband was born to nonconsanguineous healthy Subsequently, ~20 cases of pathogenic homozygous or parents at 38 weeks gestational age after unremarkable compound heterozygous ACO2 variants have been delivery. -

The Potential for Transition Metal-Mediated Neurodegeneration in Amyotrophic Lateral Sclerosis
REVIEW ARTICLE published: 23 July 2014 AGING NEUROSCIENCE doi: 10.3389/fnagi.2014.00173 The potential for transition metal-mediated neurodegeneration in amyotrophic lateral sclerosis David B. Lovejoy* and Gilles J. Guillemin Australian School of Advanced Medicine, Macquarie University, Sydney, NSW, Australia Edited by: Modulations of the potentially toxic transition metals iron (Fe) and copper (Cu) are impli- Roger S. Chung, Macquarie cated in the neurodegenerative process in a variety of human disease states including University, USA amyotrophic lateral sclerosis (ALS). However, the precise role played by these metals is Reviewed by: Junming Wang, University of still very much unclear, despite considerable clinical and experimental data suggestive of Mississippi Medical Center, USA a role for these elements in the neurodegenerative process.The discovery of mutations in Ramon Santos El-Bachá, Universidade the antioxidant enzyme Cu/Zn superoxide dismutase 1 (SOD-1) in ALS patients established Federal da Bahia, Brazil the first known cause of ALS. Recent data suggest that various mutations in SOD-1 affect *Correspondence: metal-binding of Cu and Zn, in turn promoting toxic protein aggregation. Copper home- David B. Lovejoy, Macquarie University, Australian School of ostasis is also disturbed in ALS, and may be relevant to ALS pathogenesis. Another set Advanced Medicine, Motor Neuron of interesting observations in ALS patients involves the key nutrient Fe. In ALS patients, and Neurodegenerative Diseases Fe loading can be inferred by studies showing increased expression of serum ferritin, an Research Group, Building F10A, 2 Fe-storage protein, with high serum ferritin levels correlating to poor prognosis. Magnetic Technology Place, NSW, 2109, Australia resonance imaging of ALS patients shows a characteristic T2 shortening that is attributed e-mail: [email protected] to the presence of Fe in the motor cortex. -

Mitochondria in Hematopoiesis and Hematological Diseases
Oncogene (2006) 25, 4757–4767 & 2006 Nature Publishing Group All rights reserved 0950-9232/06 $30.00 www.nature.com/onc REVIEW Mitochondria in hematopoiesis and hematological diseases M Fontenay1, S Cathelin2, M Amiot3, E Gyan1 and E Solary2 1Inserm U567, Institut Cochin, Department of Hematology, Paris, Cedex, France; 2Inserm U601, Biology Institute, Nantes, Cedex, France and 3Inserm U517, Faculty of Medicine, Dijon, France Mitochondria are involved in hematopoietic cell homeo- Introduction stasis through multiple ways such as oxidative phosphor- ylation, various metabolic processes and the release of As in other tissues, mitochondria play many important cytochrome c in the cytosol to trigger caspase activation roles in hematopoietic cell homeostasis, including the and cell death. In erythroid cells, the mitochondrial steps production of adenosine triphosphate (ATP) by the in heme synthesis, iron (Fe) metabolism and Fe-sulfur process of oxidative phosphorylation, the release of (Fe-S) cluster biogenesis are of particular importance. death-promoting factors upon apoptotic stimuli and a Mutations in the specific d-aminolevulinic acid synthase variety of metabolic pathways such as heme synthesis. (ALAS) 2 isoform that catalyses the first and rate-limiting Mitochondria could also play a role in specific pathways step in heme synthesis pathway in the mitochondrial of hematopoietic cell differentiation through caspase matrix, lead to ineffective erythropoiesis that charac- activation.Alterations of these mitochondrial functions terizes X-linked -
![(ACO2) Mouse Monoclonal Antibody [Clone ID: OTI7G4] Product Data](https://docslib.b-cdn.net/cover/3055/aco2-mouse-monoclonal-antibody-clone-id-oti7g4-product-data-823055.webp)
(ACO2) Mouse Monoclonal Antibody [Clone ID: OTI7G4] Product Data
OriGene Technologies, Inc. 9620 Medical Center Drive, Ste 200 Rockville, MD 20850, US Phone: +1-888-267-4436 [email protected] EU: [email protected] CN: [email protected] Product datasheet for TA500873 Aconitase 2 (ACO2) Mouse Monoclonal Antibody [Clone ID: OTI7G4] Product data: Product Type: Primary Antibodies Clone Name: OTI7G4 Applications: FC, IF, WB Recommended Dilution: WB 1:2000, IF 1:100, Flow 1:100 Reactivity: Human, Mouse, Rat Host: Mouse Isotype: IgG1 Clonality: Monoclonal Immunogen: Full length human recombinant protein of human ACO2 (NP_001089) produced in HEK293T cell. Formulation: PBS (pH 7.3) containing 1% BSA, 50% glycerol and 0.02% sodium azide. Concentration: 1 mg/ml Purification: Purified from mouse ascites fluids or tissue culture supernatant by affinity chromatography (protein A/G) Conjugation: Unconjugated Storage: Store at -20°C as received. Stability: Stable for 12 months from date of receipt. Predicted Protein Size: 85.4 kDa Gene Name: aconitase 2 Database Link: NP_001089 Entrez Gene 11429 MouseEntrez Gene 79250 RatEntrez Gene 50 Human Q99798 Background: The protein encoded by this gene belongs to the aconitase/IPM isomerase family. It is an enzyme that catalyzes the interconversion of citrate to isocitrate via cis-aconitate in the second step of the TCA cycle. This protein is encoded in the nucleus and functions in the mitochondrion. It was found to be one of the mitochondrial matrix proteins that are preferentially degraded by the serine protease 15(PRSS15), also known as Lon protease, after oxidative modification. This product is to be used for laboratory only. Not for diagnostic or therapeutic use.