Lassa Virus Targeting of Anterior Uvea and Endothelium of Cornea and Conjunctiva in Eye of Guinea Pig Model Joy M
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Symptoms of Age Related Macular Degeneration
WHAT IS MACULAR DEGENERATION? wavy or crooked, visual distortions, doorway and the choroid are interrupted causing waste or street signs seem bowed, or objects may deposits to form. Lacking proper nutrients, the light- Age related macular degeneration (AMD) is appear smaller or farther away than they sensitive cells of the macula become damaged. a disease that may either suddenly or gradually should, decrease in or loss of central vision, and The damaged cells can no longer send normal destroy the macula’s ability to maintain sharp, a central blurry spot. signals from the macula through the optic nerve to central vision. Interestingly, one’s peripheral or DRY: Progression with dry AMD is typically slower your brain, and consequently your vision becomes side vision remains unaffected. AMD is the leading de-gradation of central vision: need for increasingly blurred cause of “legal blindness” in the United States for bright illumination for reading or near work, diffi culty In either form of AMD, your vision may remain fi ne persons over 65 years of age. AMD is present in adapting to low levels of illumination, worsening blur in one eye up to several years even while the other approximately 10 percent of the population over of printed words, decreased intensity or brightness of eye’s vision has degraded. Most patients don’t the age of 52 and in up to 33 percent of individuals colors, diffi culty recognizing faces, gradual increase realize that one eye’s vision has been severely older than 75. The macula allows alone gives us the in the haziness of overall vision, and a profound drop reduced because your brain compensates the bad ability to have: sharp vision, clear vision, color vision, in your central vision acuity. -

Endothelium Ii
Br J Ophthalmol: first published as 10.1136/bjo.42.11.667 on 1 November 1958. Downloaded from Brit. J. Ophthal. (1958) 42, 667. STUDIES ON THE CORNEAL AND TRABECULAR ENDOTHELIUM II. ENDOTHELIUM OF THE ZONE OF TRANSITION* BY F. VRABEC From the First Eye Clinic, University ofPrague, Czechoslovakia AT the periphery of the cornea the corneal endothelium passes over the margin of Descemet's membrane to the trabecular meshwork (Vrabec, 1957). The size and shape of the cells of the corneal endothelium undergo a peculiar change in approaching this region (Vrabec, 1958a). A study by means of the replica method (Vrabec, 1958b) demonstrated that the endothelial cells became elongated in the meridional direction and then lost their outlines in the region of the anterior border of Schwalbe's ring. Only a few nuclei were seen by the replica technique. The results of examining the endothelium of this zone of transition by various methods is described below. copyright. Material and Methods The eyes of the cat, rabbit, and rhesus monkey were studied, together with some human eyes with different pathological conditions, and one human eye which was clinically niormal but was enucleated because of an orbital tumour. The basic method used was again the silver impregnation method of McGovern (1955, 1956). The results were compared with those obtained by the replica and pseudo-replica methods, the latter mostly with additional staining. Even in the http://bjo.bmj.com/ apparently normal eye, the possibility of functional changes in the secretion of the cement substance as well as of the covering substance should be borne in mind. -

Corneal Endothelium Endothelium Cells Are Destroyed by Disease Or Trauma, Thelial Cells Per Year
Integrating the Best Davis EyeCare Technology Associates Specular Microscopy is a new tech- nique to monitor corneal cell loss due to damage from extended con- tact lens wear, surgery, or the ag- ing process. It is also an excellent tool for: educating patients, screening for corneal disease (fuchs-guttata, kerataconus, Corneal trauma, dry eye, glaucoma, diabe- tes and certain medications etc.) and observing the damaging ef- Endothelium fects of contact lens wear. Often we can avoid more serious complica- tions of con- tact lens wear by under- standing the condition of the cornea. In early stages simply ad- justing the wearing time or chang- ing to a different contact lens ma- terial avoids future issues. In our elderly population our cell counts diminish and with a specular mi- croscope we can monitor and treat Davis EyeCare Associates the aging cornea much more effec- tively. This is an essential tool in managing our contact lens patients 4663 West 95th Street www.daviseyecare.com and is very important in assessing Oak Lawn Il 60453 potential risks for cataracts and Phone: 708-636-0600 4663 West 95th Street refractive surgery. Oak Lawn IL 60453 Fax: 708-636-0606 708-636-0600 E-mail: www.daviseyecare.com mal aging, the central cornea loses 100 to 500 endo- Corneal Endothelium endothelium cells are destroyed by disease or trauma, thelial cells per year. When these cells die, they they are lost forever. slough off the posterior surface of the cornea into the We are pleased to offer Konan Microscopy to the anterior chamber, creating a gap in the endothelial Common ocular conditions, such as glaucoma, uveitis management of your eyecare. -

Tissue Engineering of the Corneal Endothelium: a Review of Carrier Materials
J. Funct. Biomater. 2013, 4, 178-208; doi:10.3390/jfb4040178 Journal of Functional Biomaterials ISSN 2079-4983 www.mdpi.com/journal/jfb/ Review Tissue Engineering of the Corneal Endothelium: A Review of Carrier Materials Juliane Teichmann 1,2,†, Monika Valtink 2,†,*, Mirko Nitschke 1, Stefan Gramm 1, Richard H.W. Funk 2,3, Katrin Engelmann 3,4 and Carsten Werner 1,3 1 Leibniz Institute of Polymer Research Dresden, Max Bergmann Center of Biomaterials, Institute of Biofunctional Polymer Materials, Hohe Straße 6, Dresden 01069, Germany; E-Mails: [email protected] (J.T.); [email protected] (M.N.); [email protected] (S.G.); [email protected] (C.W.) 2 Institute of Anatomy, Medical Faculty Carl Gustav Carus, Technische Universität Dresden, Fetscherstraße 74, Dresden 01307, Germany; E-Mail: [email protected] 3 CRTD/DFG-Center for Regenerative Therapies Dresden—Cluster of Excellence, Fetscherstraße 105, Dresden 01307, Germany 4 Department of Ophthalmology, Klinikum Chemnitz gGmbH, Flemmingstraße 2, Chemnitz 09116, Germany; E-Mail: [email protected] † These authors contribute equally to the work. * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +49-351-458-6124; Fax: +49-351-458-6303. Received: 3 August 2013; in revised form: 13 September 2013 / Accepted: 24 September 2013 / Published: 22 October 2013 Abstract: Functional impairment of the human corneal endothelium can lead to corneal blindness. In order to meet the high demand for transplants with an appropriate human corneal endothelial cell density as a prerequisite for corneal function, several tissue engineering techniques have been developed to generate transplantable endothelial cell sheets. -

The Eye Is a Natural Optical Tool
KEY CONCEPT The eye is a natural optical tool. BEFORE, you learned NOW, you will learn •Mirrors and lenses focus light • How the eye depends on to form images natural lenses •Mirrors and lenses can alter • How artificial lenses can be images in useful ways used to correct vision problems VOCABULARY EXPLORE Focusing Vision cornea p. 607 How does the eye focus an image? pupil p. 607 retina p. 607 PROCEDURE 1 Position yourself so you can see an object about 6 meters (20 feet) away. 2 Close one eye, hold up your index finger, and bring it as close to your open eye as you can while keeping the finger clearly in focus. 3 Keeping your finger in place, look just to the side at the more distant object and focus your eye on it. 4 Without looking away from the more distant object, observe your finger. WHAT DO YOU THINK? • How does the nearby object look when you are focusing on something distant? • What might be happening in your eye to cause this change in the nearby object? The eye gathers and focuses light. The eyes of human beings and many other animals are natural optical tools that process visible light. Eyes transmit light, refract light, and respond to different wavelengths of light. Eyes contain natural lenses that focus images of objects. Eyes convert the energy of light waves into signals that can be sent to the brain. The brain interprets these signals as shape, brightness, and color. Altogether, these processes make vision possible. In this section, you will learn how the eye works. -

Management of Post Operative Seclusio Pupil and Corneal Decompensation
MedDocs Publishers Annals of Ophthalmology and Visual Sciences Open Access | Case Report Management of post operative seclusio pupil and corneal decompensation Arjun Srirampur, MS, FRCS*; Kavya Reddy, MS; Aruna Kumari Gadde, MS; Sunny Manwani, DNB Anand Eye Institue, Habsiguda, Hyderabad, India *Corresponding Author(s): Arjun Srirampur, Abstract Anand Eye Institue, Habsiguda, Hyderabad, India A 71 year old man presented with a history of cataract Email: [email protected] surgery in both eyes and gradual diminution of vision post surgery in right eye (RE) since 3 years. On examination RE showed corneal decompensation with seclusio pupillae Received: Jan 25, 2018 with a small pupil and a posterior chamber intraocular lens (PCIOL). He was diagnosed with pseudophakic bullous ker- Accepted: Apr 20, 2018 atopathy (PBK). He underwent DSAEK (Descemet’s stripping Published Online: Apr 27, 2018 automated endothelial keratoplasty ) with synechiolysis and Journal: Annals of Ophthalmology and Visual Sciences pupilloplasty. Graft lenticule was well attached to the host Publisher: MedDocs Publishers LLC tissue with a vertically oval pupil and subsequent improve- ment of vision. Online edition: http://meddocsonline.org/ Copyright: © Srirampur A (2018). This Article is distributed under the terms of Creative Commons Attribution 4.0 international License Keywords: Seclusio pupil; Corneal decompensation ; De- scemet’s stripping automated endothelial keratoplasty Introduction More recently, long-term follow-up has revealed the exis- tence of progressive changes in corneal endothelium following PBK may occur in around 1 to 2% of the patients undergo- intraocular lens insertion. Though the pathogenesis of this phe- ing cataract surgery, which accounts two to four million patients nomenon is not clear, persistent low grade inflammation and worldwide. -
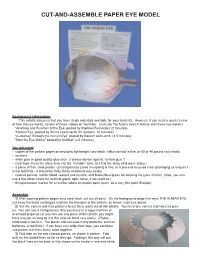
Cut-And-Assemble Paper Eye Model
CUT-AND-ASSEMBLE PAPER EYE MODEL Background information: This activity assumes that you have study materials available for your students. However, if you need a quick review of how the eye works, try one of these videos on YouTube. (Just use YouTube’s search feature with these key words.) “Anatomy and Function of the Eye: posted by Raphael Fernandez (2 minutes) “Human Eye” posted by Smart Learning for All (cartoon, 10 minutes) “A Journey Through the Human Eye” posted by Bausch and Lomb (2.5 minutes) “How the Eye Works” posted by AniMed (2.5 minutes) You will need: • copies of the pattern pages printed onto lightweight card stock (vellum bristol is fine, or 65 or 90 pound card stock) • scissors • white glue or good quality glue stick (I always advise against “school glue.”) • clear tape (I use the shiny kind, not the “invisible” kind, as I find the shiny kind more sticky.) • a piece of thin, clear plastic (a transparency [used in copiers] is fine, or a piece of recycled clear packaging as long as it is not too thick-- it should be fairly flimsy and bend very easily) • colored pencils: red for blood vessels and muscle, and brown/blue/green for coloring iris (your choice) (Also, you can use a few other colors for lacrimal gland, optic nerve, if you want to.) • thin permanent marker for a number labels on plastic parts (such as a very thin point Sharpie) Assembly: 1) After copying pattern pages onto card stock, cut out all parts. On the background page that says THE HUMAN EYE, cut away the black rectangles and trim the triangles at the bottom, as shown in picture above. -

Scleral Lenses and Eye Health
Scleral Lenses and Eye Health Anatomy and Function of the Human Eye How Scleral Lenses Interact with the Ocular Surface Just as the skin protects the human body, the ocular surface protects the human Scleral lenses are large-diameter lenses designed to vault the cornea and rest on the conjunctival tissue sitting on eye. The ocular surface is made up of the cornea, the conjunctiva, the tear film, top of the sclera. The space between the back surface of the lens and the cornea acts as a fluid reservoir. Scleral and the glands that produce tears, oils, and mucus in the tear film. lenses can range in size from 13mm to 19mm, although larger diameter lenses may be designed for patients with more severe eye conditions. Due to their size, scleral lenses consist SCLERA: The sclera is the white outer wall of the eye. It is SCLERAL LENS made of collagen fibers that are arranged for strength rather of at least two zones: than transmission of light. OPTIC ZONE The optic zone vaults over the cornea CORNEA: The cornea is the front center portion of the outer Cross section of FLUID RESERVOIR wall of the eye. It is made of collagen fibers that are arranged in the eye shows The haptic zone rests on the conjunctiva such a way so that the cornea is clear. The cornea bends light the cornea, overlying the sclera as it enters the eye so that the light is focused on the retina. conjunctiva, and sclera as CORNEA The cornea has a protective surface layer called the epithelium. -

Anatomy and Physiology of the Afferent Visual System
Handbook of Clinical Neurology, Vol. 102 (3rd series) Neuro-ophthalmology C. Kennard and R.J. Leigh, Editors # 2011 Elsevier B.V. All rights reserved Chapter 1 Anatomy and physiology of the afferent visual system SASHANK PRASAD 1* AND STEVEN L. GALETTA 2 1Division of Neuro-ophthalmology, Department of Neurology, Brigham and Womens Hospital, Harvard Medical School, Boston, MA, USA 2Neuro-ophthalmology Division, Department of Neurology, Hospital of the University of Pennsylvania, Philadelphia, PA, USA INTRODUCTION light without distortion (Maurice, 1970). The tear–air interface and cornea contribute more to the focusing Visual processing poses an enormous computational of light than the lens does; unlike the lens, however, the challenge for the brain, which has evolved highly focusing power of the cornea is fixed. The ciliary mus- organized and efficient neural systems to meet these cles dynamically adjust the shape of the lens in order demands. In primates, approximately 55% of the cortex to focus light optimally from varying distances upon is specialized for visual processing (compared to 3% for the retina (accommodation). The total amount of light auditory processing and 11% for somatosensory pro- reaching the retina is controlled by regulation of the cessing) (Felleman and Van Essen, 1991). Over the past pupil aperture. Ultimately, the visual image becomes several decades there has been an explosion in scientific projected upside-down and backwards on to the retina understanding of these complex pathways and net- (Fishman, 1973). works. Detailed knowledge of the anatomy of the visual The majority of the blood supply to structures of the system, in combination with skilled examination, allows eye arrives via the ophthalmic artery, which is the first precise localization of neuropathological processes. -

Corneal Erosion?
What Is the Cornea? The cornea is the clear front window of the eye. It covers the iris (colored portion of the eye) and the round pupil, much like a watch crystal covers the face of a watch. The cornea is composed of five layers. The outermost surface layer is called the epithelium. Normal Eye Anatomy What Is a Corneal Abrasion? A corneal abrasion is an injury (a scratch, scrape or cut) to the corneal epithelium. Abrasions are commonly caused by fingernail scratches, paper cuts, makeup brushes, scrapes from tree or bush limbs, and rubbing of the eye. Some eye conditions, such as dry eye, increase the chance of an abrasion. You may experience the following symptoms with corneal abrasion: • Feeling of having something in your eye • Pain and soreness of the eye • Redness of the eye • Sensitivity to light • Tearing • Blurred vision To detect an abrasion on the cornea, your ophthalmologist (Eye M.D.) will use a special dye called fluorescein (pronounced FLOR-uh-seen) to illuminate the injury. How Is a Corneal Abrasion Treated? Treatment may include the following: • Patching the injured eye to prevent eyelid blinking from irritating the injury. • Applying lubricating eyedrops or ointment to the eye to form a soothing layer between the eyelid and the abrasion. • Using antibiotics to prevent infection. • Dilating (widening) the pupil to relieve pain. • Wearing a special contact lens to help healing. Minor abrasions usually heal within a day or two; larger abrasions usually take about a week. It is important not to rub the eye while it is healing. -

Corneal Endothelium: Developmental Strategies for Regeneration
Eye (2013) 27, 579–588 & 2013 Macmillan Publishers Limited All rights reserved 0950-222X/13 www.nature.com/eye 1 2 Corneal J Zavala ,GRLo´ pez Jaime , REVIEW CA Rodrı´guez Barrientos1 and J Valdez-Garcia1;3 endothelium: developmental strategies for regeneration Abstract The main treatment available for restoration of culture’, ‘mesenchymal stem cells AND cell the corneal endothelium is keratoplasty. This therapy’, ‘mesenchymal stem cells AND procedure is faced with several difficulties, cornea’, and ‘stem cells AND (cornea OR including the shortage of donor tissue, post- corneal) AND (endothelial OR endothelium)’. surgical complications associated with the use Eye (2013) 27, 579–588; doi:10.1038/eye.2013.15; of drugs to prevent immune rejection, and a published online 8 March 2013 significant increase in the occurrence of glau- coma. Recently, surgical procedures such as Keywords: corneal endothelium; tissue Descemet’s stripping endothelial keratoplasty engineer; stems cells have focused on the transplant of corneal endothelium, yielding better visual results but 1Ophthalmology Research still facing the need for donor tissue. The Chair, Tecnologico de emergent strategies in the field of cell biology Monterrey, School of and tissue cultivation of corneal endothelial Medicine and Health Introduction cells aim at the production of transplantable Sciences, Monterrey, Me´ xico endothelial cell sheets. Cell therapy focuses on The cornea is a transparent avascular tissue that 2Medical and Surgical Retina the culture of corneal endothelial cells in conjunction with the sclera forms the outer Residency Program retrieved from the donor, in the donor’s portion of the eye. It is a connective tissue that Department, Universidad de cornea, followed by transplantation into the acts as the primary barrier against infection and Guadalajara Instituto de recipient. -

Physiological and Pathobiological Significance of Ocular Glycoproteins
Br J Ophthalmol: first published as 10.1136/bjo.69.3.162 on 1 March 1985. Downloaded from British Journal ofOphthalmology, 1985,69, 162-170 Physiological and pathobiological significance of ocular glycoproteins. I. Studies using fluorescein labelled glycine max A I AHMED AND A H S RAHI From the Department of Pathology, Institute of Ophthalmology, 17/25 Cayton Street, London ECI V 9A T SUMMARY Cell surface carbohydrates play an important role in several biological, immunological, and neoplastic phenomena including development, growth regulation, cellular locomotion, receptor activation, and tumour metastasis. Fluorescein labelled lectins which bind to specific carbohydrate residues in glycoproteins and glycolipids are being increasingly used as chemical probes to study cell components. Several different preparations of ocular tissues from human, rabbit, and rat were examined for the distribution of N-acetyl-D-galactosamine (D-gal NAc) by means of fluorescein-labelled lectin from soybean (glycine max). A very strong fluorescence was observed in the corneal epithelium; Descemet's membrane and corneal endothelium were also strongly fluorescent. The conjunctival epithelium similarly showed a strong reaction, as did the goblet cells. The iris epithelium and the dilator pupillae were only weakly fluorescent, but the ciliary body showed strong fluorescence, as did the blood vessels. As compared with lens fibres the lens epithelium was strongly fluorescent. The outer retina, that is, the photoreceptors, the pigment epithelium, and Bruch's membrane, showed a very strong reactivity. The optic nerve showed moderate fluorescence, but reaction with extraocular muscles was variable. The skin of the upper and lower eyelids, hair follicles, and blood vessels showed strong lectin binding.