"Electrochemistry in Nonaqueous Solutions"
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Kinetic and Equilibrium Acidities of Nitrocycloalkanes J
Kinetic and Equilibrium Acidities of Nitrocycloalkanes J. Org. Chem., Vol. 43, No. 16, 1978 3113 Acknowledgment. We are grateful to the National Science 13) M. Eigen, Angew, Chem., lnt. Ed. Engl., 3, l(1964). 14) E. Grunwald and D. Eustace, ref IO, Chapter 4,have shown that usually Foundation and the National Institutes of Health for support one or two water molecules separate the reactants in proton transfers of of this research. Comments of a referee and of Professor A. J. this type. Kresge were helpful in preparing a revised version of this 15) R. A. More O’Ferrall, ref IO, Chapter 8. 16) R. A. Ogg and M. Polyani, Trans. Faraday Soc., 31, 604 (1935). manuscript. 17) E. A. Moelwyn-Hughes and D. Glen, Roc. R. SOC.London, Ser. A,, 212, 260 (1952). References and Notes (18) C. D. Ritchie, “Solute-Solvent Interations”, J. F. Coetzee and C. 0. Ritchie, Eds., Marcel Dekker, New York, N.Y., 1969,Chapter 4,pp 266-268. (1) National Institutes of Health Fellow, 1967-1970. (19) R. A. Marcus, J. Phys. Chem., 72, 891 (1968). (2) R. P. Bell and D. M. Goodall, Proc. R. Soc.London, Ser. A, 294, 273 (1966); (20)Hydroxide (or alkoxide) ions are believed to be solvated by three tightly D. J. Barnes and R. P. Bell, ibid., 318,421 (1970);R. P. Bell and B. J. Cox, bound (inner sphere) water molecules. [The additional (outer sphere) water J. Chem. SOC.6, 194 (1970). molecules that are also present are not shown.] Judging from the energy (3) V. -

Development Team
Paper No: 16 Environmental Chemistry Module: 01 Environmental Concentration Units Development Team Prof. R.K. Kohli Principal Investigator & Prof. V.K. Garg & Prof. Ashok Dhawan Co- Principal Investigator Central University of Punjab, Bathinda Prof. K.S. Gupta Paper Coordinator University of Rajasthan, Jaipur Prof. K.S. Gupta Content Writer University of Rajasthan, Jaipur Content Reviewer Dr. V.K. Garg Central University of Punjab, Bathinda Anchor Institute Central University of Punjab 1 Environmental Chemistry Environmental Environmental Concentration Units Sciences Description of Module Subject Name Environmental Sciences Paper Name Environmental Chemistry Module Name/Title Environmental Concentration Units Module Id EVS/EC-XVI/01 Pre-requisites A basic knowledge of concentration units 1. To define exponents, prefixes and symbols based on SI units 2. To define molarity and molality 3. To define number density and mixing ratio 4. To define parts –per notation by volume Objectives 5. To define parts-per notation by mass by mass 6. To define mass by volume unit for trace gases in air 7. To define mass by volume unit for aqueous media 8. To convert one unit into another Keywords Environmental concentrations, parts- per notations, ppm, ppb, ppt, partial pressure 2 Environmental Chemistry Environmental Environmental Concentration Units Sciences Module 1: Environmental Concentration Units Contents 1. Introduction 2. Exponents 3. Environmental Concentration Units 4. Molarity, mol/L 5. Molality, mol/kg 6. Number Density (n) 7. Mixing Ratio 8. Parts-Per Notation by Volume 9. ppmv, ppbv and pptv 10. Parts-Per Notation by Mass by Mass. 11. Mass by Volume Unit for Trace Gases in Air: Microgram per Cubic Meter, µg/m3 12. -

The Vertical Distribution of Soluble Gases in the Troposphere
UC Irvine UC Irvine Previously Published Works Title The vertical distribution of soluble gases in the troposphere Permalink https://escholarship.org/uc/item/7gc9g430 Journal Geophysical Research Letters, 2(8) ISSN 0094-8276 Authors Stedman, DH Chameides, WL Cicerone, RJ Publication Date 1975 DOI 10.1029/GL002i008p00333 License https://creativecommons.org/licenses/by/4.0/ 4.0 Peer reviewed eScholarship.org Powered by the California Digital Library University of California Vol. 2 No. 8 GEOPHYSICAL RESEARCHLETTERS August 1975 THE VERTICAL DISTRIBUTION OF SOLUBLE GASES IN THE TROPOSPHERE D. H. Stedman, W. L. Chameides, and R. J. Cicerone Space Physics Research Laboratory Department of Atmospheric and Oceanic Science The University of Michigan, Ann Arbor, Michigan 48105 Abstrg.ct. The thermodynamic properties of sev- Their arg:uments were based on the existence of an eral water-soluble gases are reviewed to determine HC1-H20 d•mer, but we consider the conclusions in- the likely effect of the atmospheric water cycle dependeht of the existence of dimers. For in- on their vertical profiles. We find that gaseous stance, Chameides[1975] has argued that HNO3 HC1, HN03, and HBr are sufficiently soluble in should follow the water-vapor mixing ratio gradi- water to suggest that their vertical profiles in ent, based on the high solubility of the gas in the troposphere have a similar shape to that of liquid water. In this work, we generalize the water va•or. Thuswe predict that HC1,HN03, and moredetailed argumentsof Chameides[1975] and HBr exhibit a steep negative gradient with alti- suggest which species should exhibit this nega- tude roughly equal to the altitude gradient of tive gradient and which should not. -

Package 'Marelac'
Package ‘marelac’ February 20, 2020 Version 2.1.10 Title Tools for Aquatic Sciences Author Karline Soetaert [aut, cre], Thomas Petzoldt [aut], Filip Meysman [cph], Lorenz Meire [cph] Maintainer Karline Soetaert <[email protected]> Depends R (>= 3.2), shape Imports stats, seacarb Description Datasets, constants, conversion factors, and utilities for 'MArine', 'Riverine', 'Estuarine', 'LAcustrine' and 'Coastal' science. The package contains among others: (1) chemical and physical constants and datasets, e.g. atomic weights, gas constants, the earths bathymetry; (2) conversion factors (e.g. gram to mol to liter, barometric units, temperature, salinity); (3) physical functions, e.g. to estimate concentrations of conservative substances, gas transfer and diffusion coefficients, the Coriolis force and gravity; (4) thermophysical properties of the seawater, as from the UNESCO polynomial or from the more recent derivation based on a Gibbs function. License GPL (>= 2) LazyData yes Repository CRAN Repository/R-Forge/Project marelac Repository/R-Forge/Revision 205 Repository/R-Forge/DateTimeStamp 2020-02-11 22:10:45 Date/Publication 2020-02-20 18:50:04 UTC NeedsCompilation yes 1 2 R topics documented: R topics documented: marelac-package . .3 air_density . .5 air_spechum . .6 atmComp . .7 AtomicWeight . .8 Bathymetry . .9 Constants . 10 convert_p . 11 convert_RtoS . 11 convert_salinity . 13 convert_StoCl . 15 convert_StoR . 16 convert_T . 17 coriolis . 18 diffcoeff . 19 earth_surf . 21 gas_O2sat . 23 gas_satconc . 25 gas_schmidt . 27 gas_solubility . 28 gas_transfer . 31 gravity . 33 molvol . 34 molweight . 36 Oceans . 37 redfield . 38 ssd2rad . 39 sw_adtgrad . 40 sw_alpha . 41 sw_beta . 42 sw_comp . 43 sw_conserv . 44 sw_cp . 45 sw_dens . 47 sw_depth . 49 sw_enthalpy . 50 sw_entropy . 51 sw_gibbs . 52 sw_kappa . -

Calculation Exercises with Answers and Solutions
Atmospheric Chemistry and Physics Calculation Exercises Contents Exercise A, chapter 1 - 3 in Jacob …………………………………………………… 2 Exercise B, chapter 4, 6 in Jacob …………………………………………………… 6 Exercise C, chapter 7, 8 in Jacob, OH on aerosols and booklet by Heintzenberg … 11 Exercise D, chapter 9 in Jacob………………………………………………………. 16 Exercise E, chapter 10 in Jacob……………………………………………………… 20 Exercise F, chapter 11 - 13 in Jacob………………………………………………… 24 Answers and solutions …………………………………………………………………. 29 Note that approximately 40% of the written exam deals with calculations. The remainder is about understanding of the theory. Exercises marked with an asterisk (*) are for the most interested students. These exercises are more comprehensive and/or difficult than questions appearing in the written exam. 1 Atmospheric Chemistry and Physics – Exercise A, chap. 1 – 3 Recommended activity before exercise: Try to solve 1:1 – 1:5, 2:1 – 2:2 and 3:1 – 3:2. Summary: Concentration Example Advantage Number density No. molecules/m3, Useful for calculations of reaction kmol/m3 rates in the gas phase Partial pressure Useful measure on the amount of a substance that easily can be converted to mixing ratio Mixing ratio ppmv can mean e.g. Concentration relative to the mole/mole or partial concentration of air molecules. Very pressure/total pressure useful because air is compressible. Ideal gas law: PV = nRT Molar mass: M = m/n Density: ρ = m/V = PM/RT; (from the two equations above) Mixing ratio (vol): Cx = nx/na = Px/Pa ≠ mx/ma Number density: Cvol = nNav/V 26 -1 Avogadro’s -

Lecture 3. the Basic Properties of the Natural Atmosphere 1. Composition
Lecture 3. The basic properties of the natural atmosphere Objectives: 1. Composition of air. 2. Pressure. 3. Temperature. 4. Density. 5. Concentration. Mole. Mixing ratio. 6. Gas laws. 7. Dry air and moist air. Readings: Turco: p.11-27, 38-43, 366-367, 490-492; Brimblecombe: p. 1-5 1. Composition of air. The word atmosphere derives from the Greek atmo (vapor) and spherios (sphere). The Earth’s atmosphere is a mixture of gases that we call air. Air usually contains a number of small particles (atmospheric aerosols), clouds of condensed water, and ice cloud. NOTE : The atmosphere is a thin veil of gases; if our planet were the size of an apple, its atmosphere would be thick as the apple peel. Some 80% of the mass of the atmosphere is within 10 km of the surface of the Earth, which has a diameter of about 12,742 km. The Earth’s atmosphere as a mixture of gases is characterized by pressure, temperature, and density which vary with altitude (will be discussed in Lecture 4). The atmosphere below about 100 km is called Homosphere. This part of the atmosphere consists of uniform mixtures of gases as illustrated in Table 3.1. 1 Table 3.1. The composition of air. Gases Fraction of air Constant gases Nitrogen, N2 78.08% Oxygen, O2 20.95% Argon, Ar 0.93% Neon, Ne 0.0018% Helium, He 0.0005% Krypton, Kr 0.00011% Xenon, Xe 0.000009% Variable gases Water vapor, H2O 4.0% (maximum, in the tropics) 0.00001% (minimum, at the South Pole) Carbon dioxide, CO2 0.0365% (increasing ~0.4% per year) Methane, CH4 ~0.00018% (increases due to agriculture) Hydrogen, H2 ~0.00006% Nitrous oxide, N2O ~0.00003% Carbon monoxide, CO ~0.000009% Ozone, O3 ~0.000001% - 0.0004% Fluorocarbon 12, CF2Cl2 ~0.00000005% Other gases 1% Oxygen 21% Nitrogen 78% 2 • Some gases in Table 3.1 are called constant gases because the ratio of the number of molecules for each gas and the total number of molecules of air do not change substantially from time to time or place to place. -

Interactions Between Polymers and Nanoparticles : Formation of « Supermicellar » Hybrid Aggregates
1 Interactions between Polymers and Nanoparticles : Formation of « Supermicellar » Hybrid Aggregates J.-F. Berret@, K. Yokota* and M. Morvan, Complex Fluids Laboratory, UMR CNRS - Rhodia n°166, Cranbury Research Center Rhodia 259 Prospect Plains Road Cranbury NJ 08512 USA Abstract : When polyelectrolyte-neutral block copolymers are mixed in solutions to oppositely charged species (e.g. surfactant micelles, macromolecules, proteins etc…), there is the formation of stable “supermicellar” aggregates combining both components. The resulting colloidal complexes exhibit a core-shell structure and the mechanism yielding to their formation is electrostatic self-assembly. In this contribution, we report on the structural properties of “supermicellar” aggregates made from yttrium-based inorganic nanoparticles (radius 2 nm) and polyelectrolyte-neutral block copolymers in aqueous solutions. The yttrium hydroxyacetate particles were chosen as a model system for inorganic colloids, and also for their use in industrial applications as precursors for ceramic and opto-electronic materials. The copolymers placed under scrutiny are the water soluble and asymmetric poly(sodium acrylate)-b-poly(acrylamide) diblocks. Using static and dynamical light scattering experiments, we demonstrate the analogy between surfactant micelles and nanoparticles in the complexation phenomenon with oppositely charged polymers. We also determine the sizes and the aggregation numbers of the hybrid organic-inorganic 1 2 complexes. Several additional properties are discussed, such -

Chapter 1 Chemistry of Non-Aqueous Solutions
EFOP-3.4.3-16-2016-00014 projekt Lecture notes in English for the Chemistry of non-aqueous solutions, melts and extremely concentrated aqueous solutions (code of the course KMN131E-1) Pál Sipos University of Szeged, Faculty of Science and Informatics Institute of Chemistry Department of Inorganic and Analytical Chemistry Szeged, 2020. Cím: 6720 Szeged, Dugonics tér 13. www.u-szeged.hu www.szechenyi2020.hu EFOP-3.4.3-16-2016-00014 projekt Content Course description – aims, outcomes and prior knowledge 5 1. Chemistry of non-aqueous solutions 6 1.1 Physical properties of the molecular liquids 10 1.2 Chemical properties of the molecular liquids – acceptor and donor numbers 18 1.2.1 DN scales 18 1.2.1 AN scales 19 1.3 Classification of the solvents according to Kolthoff 24 1.4 The effect of solvent properties on chemical reactions 27 1.5 Solvation and complexation of ions and electrolytes in non-aqueous solvents 29 1.5.1 The heat of dissolution 29 1.5.2 Solvation of ions, ion-solvent interactions 31 1.5.3 The structure of the solvated ions 35 1.5.4 The effect of solvents on the complex formation 37 1.5.5 Solvation of ions in solvent mixtures 39 1.5.6 The permittivity of solvents and the association of ions 42 1.5.7 The structure of the ion-pairs 46 1.6 Acid-base reactions in non-aqueous solvents 48 1.6.1 Acid-base reactions in amphiprotic solvents of high permittivity 50 1.6.2 Acid-base reactions in aprotic solvents of high permittivity 56 1.6.3 Acid-base reactions in amphiprotic solvents of low permittivity 61 1.6.4 Acid-base reactions in amphiprotic solvents of low permittivity 61 1.7 The pH scale in non-aqueous solvents 62 1.8 Acid-base titrations in non-aqueous solvents 66 1.9 Redox reactions in non-aqueous solutions 70 2 EFOP-3.4.3-16-2016-00014 projekt 1.7.1 Potential windows of non-aqueous solvents 74 1.10 Questions and problems 77 2. -
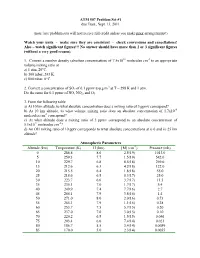
Problem Set #1 Due Tues., Sept
ATM 507 Problem Set #1 due Tues., Sept. 13, 2011 (note: late problem sets will not receive full credit unless you make prior arrangements!) Watch your units - make sure they are consistent - check conversions and cancellations! Also – watch significant figures!!! No answer should have more than 2 or 3 significant figures (without a very good reason). 1. Convert a number density (absolute concentration) of 7.5x1012 molecules cm-3 to an appropriate volume mixing ratio at a) 1 atm, 20°C. b) 100 mbar, 243 K. c) 800 mbar, 0°C. -3 2. Convert a concentration of SO2 of 0.1 ppmv to μg m at T = 298 K and 1 atm. Do the same for 0.1 ppmv of NO, NO2, and O3. 3. From the following table a) At 10 km altitude, to what absolute concentration does a mixing ratio of 3 ppmv correspond? b) At 10 km altitude, to what volume mixing ratio does an absolute concentration of 1.7x1010 molecules cm-3 correspond? c) At what altitude does a mixing ratio of 3 ppmv correspond to an absolute concentration of 5.1x1011 molecules cm-3? d) An OH mixing ratio of 10 pptv corresponds to what absolute concentrations at i) 0 and ii) 25 km altitude? Atmospheric Parameters Altitude (km) Temperature (K) H (km) [M] (cm-3) Pressure (mb) 0 288.8 8.6 2.5(19) 1013.0 5 259.3 7.7 1.5(19) 542.0 10 229.7 6.8 8.5(18) 269.0 15 212.6 6.3 4.2(18) 122.0 20 215.5 6.4 1.8(18) 55.0 25 218.6 6.5 8.3(17) 25.0 30 223.7 6.6 3.7(17) 11.5 35 235.1 7.0 1.7(17) 5.4 40 249.9 7.4 7.7(16) 2.7 45 266.1 7.9 3.8(16) 1.4 50 271.0 8.0 2.0(16) 0.73 55 265.3 7.9 1.1(16) 0.38 60 253.7 7.5 5.7(15) 0.20 65 237.0 7.0 3.0(15) 0.10 70 220.2 6.5 1.5(15) 0.046 75 203.4 6.0 7.4(14) 0.021 80 186.7 5.5 3.4(14) 0.0089 85 170.0 5.0 2.3(14) 0.0055 4. -

SI Analytics Ph Handbook
pH handbook HELPFUL GUIDE FOR PRACTICAL APPLICATION AND USE Welcome to SI Analytics! We express our core competence, namely the production of analytical instruments, with our company name SI Analytics. SI also stands for the main products of our company: sensors and instruments. As part of the history of SCHOTT® AG, SI Analytics has more than 75 years experience in glass technology and in the development of analytical equipment. As always, our products are manufactured in Mainz with a high level of innovation and quality. Our electrodes, titrators and capillary viscometers will continue to be the right tools in any location where expertise in analytical measurement technology is required. In 2011 SI Analytics became part of the listed company Xylem Inc., headquartered in Rye Brook / N.Y., USA. Xylem is a leading international provider of water solutions. We look forward to presenting the pH handbook to you! The previous publication "Interesting facts about pH measurement" has been restructured, made clearer and more engaging, and extra information has been added. The focus has been consciously put on linking general information with our lab findings and making this accessible to you in a practical format. Complemented by the reference to our range of products and with practical recommendations for use with specific applications, the pH primer is an indispensable accompaniment for everyday lab work. We at SI Analytics would be happy to work successfully together with you in the future. SI Analytics GmbH Dr. Robert Reining CEO CONTENTS SECTION 1 Basic concepts of potentiometry and pH measurement 1.1 Definition of acid and base ......................................................... -

Xerox University Microfilms 300 North Zeeb Rood Ann Arbor, Michigan 48106 11
INFORMATION TO USERS This material was produced from a microfilm copy of the original document. While the most advanced technological means to photograph and reproduce this document have been used, the quality is heavily dependent upon the quality of the original submitted. The following explanation of techniques is provided to help you understand markings or patterns which may appear on this reproduction. 1. The sign or "target" for pages apparently lacking from the document photographed is "Missing Page(s)". If it was possible to obtain the missing page(s) or section, they are spliced into the film along with adjacent pages. This may have necessitated cutting thru an image and duplicating adjacent pages to insure you complete continuity. 2. When an image^pn the film is obliterated with a large round black mark, it is an indication that the photographer suspected that the copy may have moved during exposure and thus cause a blurred image. You will find a good image of the page in the adjacent frame. 3. When a map, drawing or chart, etc., was part of the material being photographed the photographer followed a definite method in "sectioning" the material. It is customary to begin photoing at the upper left hand corner of a large sheet and to continue photoing from left to right in equal sections with a small overlap. If necessary, sectioning is continued again — beginning below the first row and continuing on until complete. 4. The majority of users indicate that the textual content is of greatest value, however, a somewhat higher quality reproduction could be made from "photographs" if essential to the understanding of the dissertation. -

Redalyc.Calcite Dissolution by Mixing Waters: Geochemical Modeling And
Geologica Acta: an international earth science journal ISSN: 1695-6133 [email protected] Universitat de Barcelona España SANZ, E.; AYORA, C.; CARRERA, J. Calcite dissolution by mixing waters: geochemical modeling and flow-through experiments Geologica Acta: an international earth science journal, vol. 9, núm. 1, marzo, 2011, pp. 67-77 Universitat de Barcelona Barcelona, España Available in: http://www.redalyc.org/articulo.oa?id=50522124007 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Geologica Acta, Vol.9, Nº 1, March 2011, 67-77 DOI: 10.1344/105.000001652 Available online at www.geologica-acta.com Calcite dissolution by mixing waters: geochemical modeling and flow-through experiments 1 2 1 E. SANZ C. AYORA J. CARRERA 1 ExxonMobil Upstream Research Company Houston, Texas, USA E-mail: [email protected] 2 Institute of Enviromental Assessment and Water Research, IDAEA-ECSIC Barcelona, Spain ABSTRACT TABLE CAPTIONS Dissolution of carbonates has been commonly predicted by geochemical models to occur at the seawater-freshwater mixing zone of coastal aquifers along a geological time scale. However, field evidences are inconclusive:dissolution TABLE 1 Input end-member solutions used in the experiments and numerical simulations. Ω accounts for saturation, and I for ionic strength. vs. lack of dissolution. In this study we investigate the process of calcite dissolution by mixing waters of different salinities and pCO2, by means of geochemical modeling and laboratory experiments.