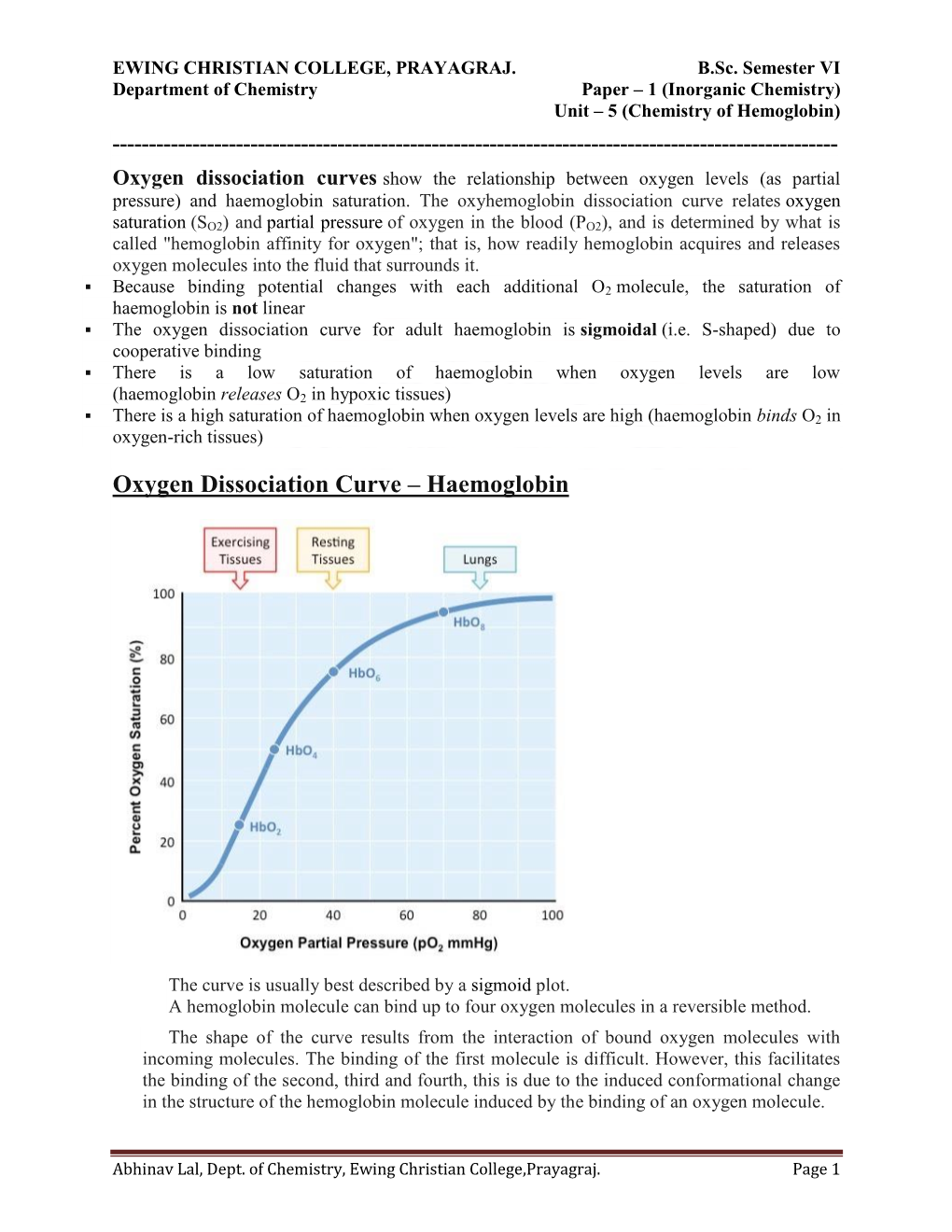
Oxygen Dissociation Curves Show the Relationship Between Oxygen Levels (As Partial Pressure) and Haemoglobin Saturation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Oxyhaemoglobin Dissociation Curve in Critical Illness
Basic sciences review The Oxyhaemoglobin Dissociation Curve in Critical Illness T. J. MORGAN Intensive Care Facility, Division of Anaesthesiology and Intensive Care, Royal Brisbane Hospital, Brisbane, QUEENSLAND ABSTRACT Objective: To review the status of haemoglobin-oxygen affinity in critical illness and investigate the potential to improve gas exchange, tissue oxygenation and outcome by manipulations of the oxyhaemoglobin dissociation curve. Data sources: Articles and published peer-review abstracts. Summary of review: The P50 of a species is determined by natural selection according to animal size, tissue metabolic requirements and ambient oxygen tension. In right to left shunting mathematical modeling indicates that an increased P50 defends capillary oxygenation, the one exception being sustained hypercapnia. Increasing the P50 should also be protective in tissue ischaemia, and this is supported by modeling and experimental evidence. Most studies of critically ill patients have indicated reduced 2,3-DPG concentrations. This is probably due to acidaemia, and the in vivo P50 is likely to be normal despite low 2,3-DPG levels. It may soon be possible to achieve significant P50 elevations without potentially harmful manipulations of acid-base balance or hazardous drug therapy. Conclusions: Despite encouraging theoretical and experimental data, it is not known whether manipulations of the P50 in critical illness can improve gas exchange and tissue oxygenation or improve outcome. The status of the P50 may warrant more routine quantification and consideration along with the traditional determinants of tissue oxygen availability. (Critical Care and Resuscitation 1999; 1: 93-100) Key words: Critical illness, haemoglobin-oxygen affinity, ischaemia, P50, tissue oxygenation, shunt In intensive care practice, manipulations to improve in the tissues. -

Pranayama Redefined/ Breathing Less to Live More
Pranayama Redefined: Breathing Less to Live More by Robin Rothenberg, C-IAYT Illustrations by Roy DeLeon ©Essential Yoga Therapy - 2017 ©Essential Yoga Therapy - 2017 REMEMBERING OUR ROOTS “When Prana moves, chitta moves. When prana is without movement, chitta is without movement. By this steadiness of prana, the yogi attains steadiness and should thus restrain the vayu (air).” Hatha Yoga Pradipika Swami Muktabodhananda Chapter 2, Verse 2, pg. 150 “As long as the vayu (air and prana) remains in the body, that is called life. Death is when it leaves the body. Therefore, retain vayu.” Hatha Yoga Pradipika Swami Muktabodhananda Chapter 2, Verse 3, pg. 153 ©Essential Yoga Therapy - 2017 “Pranayama is usually considered to be the practice of controlled inhalation and exhalation combined with retention. However, technically speaking, it is only retention. Inhalation/exhalation are methods of inducing retention. Retention is most important because is allows a longer period for assimilation of prana, just as it allows more time for the exchange of gases in the cells, i.e. oxygen and carbon dioxide.” Hatha Yoga Pradipika Swami Muktabodhananda Chapter 2, Verse 2, pg. 151 ©Essential Yoga Therapy - 2017 Yoga Breathing in the Modern Era • Focus tends to be on lengthening the inhale/exhale • Exhale to induce relaxation (PSNS activation) • Inhale to increase energy (SNS) • Big ujjayi - audible • Nose breathing is emphasized at least with inhale. Some traditions teach mouth breathing on exhale. • Focus on muscular action of chest, ribs, diaphragm, intercostals and abdominal muscles all used actively on inhale and exhale to create the ‘yoga breath.’ • Retention after inhale and exhale used cautiously and to amplify the effect of inhale and exhale • Emphasis with pranayama is on slowing the rate however no discussion on lowering volume. -

Breathing Issues: in Depth Tutorial Hypo=Low Hyper= High Or More
Breathing Issues: In Depth Tutorial Hypo=low Hyper= high or more In the normal state, when we breathe in, we take in oxygen, the oxygen goes to the lungs and gets absorbed into the circulation, in exchange for carbon dioxide. Then, we exhale the carbon dioxide. One way to think of this is that you take in oxygen and you breathe out carbon dioxide. There are two issues that can occur with breathing problems. One is a low oxygen problem (hyoxemia). The other is retention of carbon dioxide (hypercarbia). A low oxygen and a high carbon dioxide level (in lungs and blood) can sometimes be related. Pulmonologist talk about these two issues using the following terms: 1. you can have a problem with oxygenation AND/OR 2. you can have a problem with ventilation (ventilation means the process by which your lungs expand and by taking in the air you exchange the oxygen for carbon dioxide). Ventilation is the problem for people with neuromuscular disease. Ventilation problems lead to carbon dioxide build up. Ventilation requires you to have the muscle support to expand your lungs during inhalation (your chest wall muscles assist in this effort as does the diaphragm. Scoliosis can impair chest expansion). Depending on how weak you are, you may or not be able to take an effective large enough breath. Because you are not taking a deep enough breath in, when you exhale you have a smaller amount of air to breathe out and it is not enough volume to get rid of the carbon dioxide. The carbon dioxide slowly starts to build up over time. -

Improved Oxygen Release: an Adaptation of Mature Red Cells to Hypoxia
Improved oxygen release: an adaptation of mature red cells to hypoxia Miles J. Edwards, … , Carrie-Lou Walters, James Metcalfe J Clin Invest. 1968;47(8):1851-1857. https://doi.org/10.1172/JCI105875. Research Article Blood from patients with erythrocytosis secondary to arterial hypoxemia due either to congenital heart disease or to chronic obstructive pulmonary disease was shown to have a decreased affinity for oxygen; the average oxygen pressure required to produce 50% saturation of hemoglobin with oxygen was 29.8 mm Hg (average normal, 26.3 mm Hg). Such a displacement of the blood oxygen equilibrium curve promotes the release of oxygen from blood to the tissues. Studies were also performed upon blood from a man with complete erythrocyte aplasia who received all of his red cells by transfusion from presumably normal persons. With mild anemia (hematocrit, 28%), the affinity of his blood for oxygen was slightly diminished (an oxygen pressure of 27.0 mm Hg was required to produce 50% saturation of hemoglobin with oxygen). With severe anemia (hematocrit, 13.5%), however, his blood had a markedly decreased oxygen affinity (an oxygen pressure of 29.6 mm Hg was required to produce 50% saturation of hemoglobin with oxygen). We conclude that patients with various conditions characterized by an impairment in the oxygen supply system to tissues respond with a diminished affinity of their blood for oxygen. Although the mechanism which brings about this adaptation is not known, the displacement of the oxygen equilibrium curve is associated with an increase in heme-heme interaction. The decrease in blood oxygen affinity […] Find the latest version: https://jci.me/105875/pdf Improved Oxygen Release: an Adaptation of Mature Red Cells to Hypoxia MUas J. -

Respiratory System.Pdf
Respiratory System Respiratory System - Overview: Assists in the detection Protects system of odorants Respiratory (debris / pathogens / dessication) System 5 3 4 Produces sound (vocalization) Provides surface area for gas exchange (between air / blood) 1 2 For the body to survive, there must be a constant supply of Moves air to / from gas O2 and a constant exchange surface disposal of CO 2 Marieb & Hoehn (Human Anatomy and Physiology, 8th ed.) – Table 19.1 Respiratory System Respiratory System Functional Anatomy: Functional Anatomy: Trachea Epiglottis Naming of pathways: • > 1 mm diameter = bronchus Upper Respiratory • Conduction of air • < 1 mm diameter = bronchiole System • Gas exchange Primary • < 0.5 mm diameter = terminal bronchiole Bronchus • Filters / warms / humidifies Lower Respiratory Bronchi System incoming air bifurcation (23 orders) 1) External nares 5) Larynx 2) Nasal cavity • Provide open airway Green = Conducting zone • Resonance chamber • channel air / food Purple = Respiratory zone 3) Uvula • voice production (link) 4) Pharynx 6) Trachea 7) Bronchial tree • Nasopharynx Bronchiole 8) Alveoli • Oropharynx Terminal Bronchiole Respiratory Bronchiole • Laryngopharynx Alveolus Martini et. al. (Fundamentals of Anatomy and Physiology, 7th ed.) – Figure 23.1 Martini et. al. (Fundamentals of Anatomy and Physiology, 7th ed.) – Figure 23.9 Respiratory System Respiratory System Functional Anatomy: Functional Anatomy: Respiratory Mucosa / Submucosa: How are inhaled debris / pathogens cleared from respiratory tract? Near Near trachea alveoli Nasal Cavity: Epithelium: Particles > 10 µm Pseudostratified Simple columnar cuboidal Conducting Zone: Particles 5 – 10 µm Cilia No cilia Respiratory Zone: Mucus Escalator Particles 1 – 5 µm Mucosa: Lamina Propria (areolar tissue layer): Mucous membrane (epithelium / areolar tissue) smooth smooth muscle muscle Mucous No glands mucous glands Cartilage: Rings Plates / none Macrophages Martini et. -

Bohr Effect (Source: Pathway Medicine)
Bohr Effect (source: Pathway Medicine) Overview The Bohr Effect refers to the observation that increases in the carbon dioxide partial pressure of blood or decreases in blood pH result in a lower affinity of hemoglobin for oxygen. This manifests as a right-ward shift in the Oxygen-Hemoglobin Dissociation Curve described in Oxygen Transport and yields enhanced unloading of oxygen by hemoglobin. Mechanism Decreases in blood pH, meaning increased H+ concentration, are likely the direct cause of lower hemoglobin affinity for oxygen. Specifically, the association of H+ ions with the amino acids of hemoglobin appear to reduce hemoglobin's affinity for oxygen. Because changes in the carbon dioxide partial pressure can modify blood pH, increased partial pressures of carbon dioxide can also result in right-ward shifts of the oxygen-hemoglobin dissociation curve. The relationship between carbon dioxide partial pressure and blood pH is mediated by carbonic anhydrase which converts gaseous carbon dioxide to carbonic acid that in turn releases a free hydrogen ion, thus reducing the local pH of blood. Significance The Bohr Effect allows for enhanced unloading of oxygen in metabolically active peripheral tissues such as exercising skeletal muscle. Increased skeletal muscle activity results in localized increases in the partial pressure of carbon dioxide which in turn reduces the local blood pH. Because of the Bohr Effect, this results in enhanced unloading of bound oxygen by hemoglobin passing through the metabolically active tissue and thus improves oxygen delivery. Importantly, the Bohr Effect enhances oxygen delivery proportionally to the metabolic activity of the tissue. As more metabolism takes place, the carbon dioxide partial pressure increases thus causing larger reductions in local pH and in turn allowing for greater oxygen unloading. -

The Respiratory System RESPIRATORY SYSTEM
The Respiratory System RESPIRATORY SYSTEM Primary functions Major functional events Pulmonary ventilation Diffusion of O2 & CO2 between alveoli & blood Transport of O2 & CO2 in blood and body fluids Regulation of ventilation 2 Respiratory Gasses What essential function does O2 serve? How is CO2 produced and why do we need to get rid of it? 3 True or false: The oxygen you breathe in gets converted into carbon dioxide that you then breathe out. A) True B) False 4 Review of Respiratory Structures Upper vs. lower respiratory tracts Thoracic cavity Diaphragm See Fig. 37-8 5 Review of Respiratory Structures Respiratory tree Trachea Bronchi Bronchioles Alveolar sacs Alveoli 6 See Fig. 37-8 Review of Respiratory Structures Structural characteristics Cartilage Cilia Mucus glands Smooth muscle See Fig. 37-8 7 Review of Respiratory Structures Respiratory membrane Blood-air barrier Epithelial characteristics Type I cells Type II cells Produce surfactant 8 See Fig. 37-8 Review of Respiratory Structures Pleurae (membranes) Parietal pleura Visceral pleura Pleural cavity Serous fluid Lungs “float” in pleural cavities 9 Fluids in the respiratory system have all of these functions except… A) Reducing friction of lung against chest wall B) Reducing surface tension in the lung C) Allowing gasses to diffuse across epithelium D) Transporting metabolic fuels to body tissues 10 Mechanics of Breathing Boyle’s Law P1V1 = P2V2 The pressure of a gas varies inversely with its volume volume, pressure = inhalation volume, pressure = exhalation Air moves from area of high pressure to area of low pressure 11 Mechanics of Breathing Muscular events during inspiration Diaphragm contracts & flattens inferiorly External intercostals contract & elevate rib cage thoracic volume Fig. -

How Hypoxia Through Bohr Effect in Tissues and Pancreatic Beta-Cells Explains the Main Cause of T2d and T1d
HOW HYPOXIA THROUGH BOHR EFFECT IN TISSUES AND PANCREATIC BETA-CELLS EXPLAINS THE MAIN CAUSE OF T2D AND T1D The Prime Cause of type-2 (T2D), Type-1 Diabetes (T1D) And The Relation Between Diabetes and Cancer Professor Dr. Sorush Niknamian PhD in Cell and Molecular Biology, Military Medicine (Liberty University) and Board Member of Weston A Price Foundation in Washington DC, USA ABSTRACT Diabetes mellitus (DM) is a group of metabolic disorders in which there are high blood sugar levels over a prolonged period. Between 1985 and 2002, the number of people with diabetes grew from 30 million to 217 million, and this incidence will be expected to exceed 366 million by 2030. Type 1 diabetes mellitus is characterized by loss of the insulin-producing beta cells of the pancreatic islets, leading to insulin deficiency. This type can be further classified as immune- mediated or idiopathic. This research has gone through several important reviews plus one research on 21 mice which is done in Violet Cancer Institute (VCI) to find the prime reason behind T1D and T2D. We have reviewed the physiological and evolutionary mechanisms in both types of diabetes. In all cases, Hypoxia through Bohr Effect have been observed. The Bohr effect increases the efficiency of oxygen transportation through the blood. After hemoglobin binds to oxygen in the lungs because of the high oxygen concentrations, the Bohr effect facilitates its release in the tissues, specifically those tissues which need the most oxygen. Chronic hypoxia in tissues and pancreatic beta-cells through the Bohr Effect (BE) has been discussed in this review/ research as the reason for causing T2D and T1D. -

The Effect of Buteyko Breathing Technique in Improving Cardiorespiratory Endurance
ISSN: 2477-3328 The 3rd International Conference on Science, Technology, and Humanity The Effect of Buteyko Breathing Technique in Improving Cardiorespiratory Endurance Dani Fahrizal1*, Totok Budi S.1 1Universitas Muhammadiyah Surakarta, Faculty of Health Science, Pabelan Kartasura, Surakarta 57162, Indonesia *[email protected] (Dani Fahrizal) Abstract Cardiorespiratory endurance is one of the main aspects required in sports. Cardiorespiratory endurance is influenced by the function of cardiorespiratory system and body oxygenation system. Improving body oxygenation system is just as important as improving cardiorespiratory system to achieve a higher cardiorespiratory endurance. Performing a strenuous activity requires more oxygen to produce the energy. Ironically, our body adapts to a strenuous activity by increasing number of ventilation rate (hyperventilation), which leads to a decreased efficiency of body oxygenation process. Hyper-ventilation reduces carbon dioxide levels by exhaling more carbon dioxide instead of producing it. The carbon dioxide has a main role in maintaining acid-base balance. Altered pH balance causes less oxygen to be released into the tissues. Buteyko Breathing Technique is one of many breathing exercises aimed at improving body oxygenation system. It focuses on decreasing number of ventilation rate and increasing carbon dioxide levels to optimize body oxygenation process. The purpose of this research was to determine the effects of Buteyko Breathing Technique in improving cardiorespiratory endurance. 22 males aged 15-17 were involved to fulfill the objective of the study. This research used parametric test to analyze the data and to observe significant differences in VO2 max improvement between the two groups. Keywords: Buteyko Breathing Technique, Cardiorespiratory Endurance, Body Oxygenation. -

The Significance of the Bohr Effect in the Respiration and Asphyxiation of the Squid, Loligo Pealei by Alfred C
34° THE SIGNIFICANCE OF THE BOHR EFFECT IN THE RESPIRATION AND ASPHYXIATION OF THE SQUID, LOLIGO PEALEI BY ALFRED C. REDFIELD AND ROBERT GOODKIND. (From the Laboratories of Physiology of the Harvard Medical School, Boston, and the Marine Biological Laboratory, Woods Hole, Mass.) {Received Sth February 1929.) (With Three Text-figures.) IN 1904 Bohr, Hasselbalch and Krogh showed that carbon dioxide diminished the affinity of haemoglobin for oxygen. This phenomenon, known as the " Bohr effect," together with the reciprocal effect of oxygenation upon the combination of carbon dioxide with blood, discovered by Christiansen, Douglas and Haldane (1914) must be taken into account in all quantitative considerations of the respiratory function of the blood of mammals. It is now recognised that the effects of carbon dioxide are not specific, but are due to alterations in the acid-base equilibrium of the blood and may be produced by other acids. The phenomena have also been described in the case of the blood of the lower orders of vertebrates (Krogh and Leitch, 1919; and Southworth and Redfield, 1926) and of the body fluids of invertebrate animals, such as the worms which have respiratory pigments more or less closely related to haemoglobin (Barcroft and Barcroft, 1924). Recently it was shown by Redfield and Hurd (1925) that blood containing haemocyanin also exhibits the Bohr effect and that in the case of Limidus polyphemus the action of carbon dioxide is reversed. Subsequent studies by Redfield, Coolidge and Hurd (1926), Pantin and Hogben (1925), Hogben (1926), Hogben and Pinhey (1926), Stedman and Stedman (1926) have extended these observations and lead to several generalisations. -

A Biochemical−Biophysical Study of Hemoglobins from Woolly Mammoth, Asian Elephant, and Humans † † † † † Yue Yuan, Tong-Jian Shen, Priyamvada Gupta, Nancy T
Article pubs.acs.org/biochemistry A Biochemical−Biophysical Study of Hemoglobins from Woolly Mammoth, Asian Elephant, and Humans † † † † † Yue Yuan, Tong-Jian Shen, Priyamvada Gupta, Nancy T. Ho, Virgil Simplaceanu, † ‡ § ⊥ † Tsuey Chyi S. Tam, Michael Hofreiter, Alan Cooper, Kevin L. Campbell, and Chien Ho*, † Department of Biological Sciences, Carnegie Mellon University, Pittsburgh, Pennsylvania 15213, United States ‡ Department of Biology, University of York, York, YO10 5YW, United Kingdom § Australian Centre for Ancient DNA, University of Adelaide, Adelaide, SA 5005, Australia ⊥ Department of Biological Sciences, University of Manitoba, Winnipeg, Manitoba R3T 2N2, Canada *S Supporting Information ABSTRACT: This study is aimed at investigating the molecular basis of environmental adaptation of woolly mammoth hemoglobin (Hb) to the harsh thermal conditions of the Pleistocene ice ages. To this end, we have carried out a comparative biochemical−biophysical characterization of the structural and functional properties of recombinant hemoglobins (rHb) from woolly mammoth (rHb WM) and Asian elephant (rHb AE) in relation to human hemoglobins Hb A and Hb A2 (a minor component of human blood). We have obtained oxygen equilibrium curves and calculated O2 affinities, Bohr effects, and the apparent heat of oxygenation (ΔH) in the presence and absence of allosteric effectors [inorganic phosphate and inositol hexaphosphate (IHP)]. Here, we show that the four Hbs exhibit distinct structural properties and respond differently to allosteric effectors. In addition, the apparent heat of oxygenation (ΔH) for rHb WM is less negative than that of rHb AE, especially in phosphate buffer and the presence of IHP, suggesting that the oxygen affinity of mammoth blood was also less sensitive to temperature change. -

Protein Structure and Function Continued
Chemistry 5.07SC Biological Chemistry Fall Semester, 2013 I Lectures 4. Protein structure and function continued. Quaternary Structure using Hb as an example and Mb for comparison. Many proteins are composed of more than one polypeptide chain. You have already seen this with the collagen. Quaternary structure can provide a basis for regulation at a distance: allosteric regulation and cooperative behavior. We will see when studying metabolic pathways, that many of the enzymes that control the flux through the pathway (rate-limiting step(s)) have quaternary structures and exhibit cooperative behavior by binding small molecule metabolites. However, there are many other proteins composed of multiple polypeptide chains where the chains act independently. I. Big Picture: Mb and Hb have different physiological roles. Mb is a monomer and is found in tissues. It functions as a carrier of O2 to the mitochondrial in a cell. O2 has limited solubility (0.1 mM) and thus a carrier is required for rates of distribution to be sufficient for metabolism. O2 is reduced to H20 in the respiratory chain in the mitochondria and the energy released is used to make the energy currency of the cell, ATP. Hb is a tetramer composed of two types of subunits designated α and β. These subunits are structurally homologous to each other and to Mb (Figure 2). Hb is found in erythrocytes (red blood cells (RBCs)) and also functions as an O2 carrier. It acquires O2 from air through the lungs and delivers it through the circulatory system to the tissues where it is picked up by Mb.