CBW Conventions Bulletin 51
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Evaluated Gas Phase Basicities and Proton Affinities of Molecules; Heats of Formation of Protonated Molecules
Evaluated Gas Phase Basicities and Proton Affinities of Molecules; Heats of Formation of Protonated Molecules Cite as: Journal of Physical and Chemical Reference Data 13, 695 (1984); https://doi.org/10.1063/1.555719 Published Online: 15 October 2009 Sharon G. Lias, Joel F. Liebman, and Rhoda D. Levin ARTICLES YOU MAY BE INTERESTED IN Evaluated Gas Phase Basicities and Proton Affinities of Molecules: An Update Journal of Physical and Chemical Reference Data 27, 413 (1998); https:// doi.org/10.1063/1.556018 Density-functional thermochemistry. III. The role of exact exchange The Journal of Chemical Physics 98, 5648 (1993); https://doi.org/10.1063/1.464913 A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu The Journal of Chemical Physics 132, 154104 (2010); https://doi.org/10.1063/1.3382344 Journal of Physical and Chemical Reference Data 13, 695 (1984); https://doi.org/10.1063/1.555719 13, 695 © 1984 American Institute of Physics for the National Institute of Standards and Technology. Evaluated Gas Phase Basicities and Proton Affinities of Molecules; Heats of Formation of Protonated Molecules Sharon G. Lias Center for Chemical Physics, National Bureau of Standards, Gaithersburg, MD 20899 Joel F. Liebman Department of Chemistry, University of Maryland Baltimore County, Catonsville, MD 21228 and Rhoda D. Levin Center for Chemical Physics. National Bureau of Standards, Gaithersburg, MD 20899 The available data on gas phase basicities and proton affinities of molecules are compiled and evaluated. Tables giving the molecules ordered (1) according to proton affinity and (2) according to empirical formula, sorted alphabetically are provided. -

Constitutional Court of South Africa
CONSTITUTIONAL COURT OF SOUTH AFRICA Case CCT 30/03 THE STATE Applicant versus WOUTER BASSON Respondent Heard on : 4 and 5 November 2003 Decided on : 10 March 2004 JUDGMENT ACKERMANN J, MADALA J, MOKGORO J, MOSENEKE J, NGCOBO J, and O’REGAN J: [1] The state has applied to this Court for special leave to appeal against a judgment of the Supreme Court of Appeal (the SCA) in terms of rule 20 and, simultaneously, for leave to appeal directly to this Court against a judgment of the High Court in Pretoria (the High Court) in terms of rule 18. The respondent, Dr Wouter Basson, opposes both applications. Background ACKERMANN J et al [2] During 1999, the respondent, an employee of the South African National Defence Force, was charged in the High Court on 67 counts including murder, fraud, conspiracy to commit various crimes and drug offences. All the offences were allegedly committed before 1994 when the respondent worked in a division of the Defence Force called the Civil Co-operation Bureau. [3] During 1997 the accused was arrested, first on charges of contravening the Medicines and Related Substances Control Act, 101 of 1965, and later in the same year on charges of fraud. In relation to both sets of charges, bail hearings were held and the accused was granted bail. In relation to the fraud charges, the bail hearing was held during October and November 1997. The trial on all 67 charges (which now included charges of murder and conspiracy to commit various offences) commenced on 4 October 1999 before Hartzenberg J. -

Intramolecular Ene Reactions of Functionalised Nitroso Compounds
INTRAMOLECULAR ENE REACTIONS OF FUNCTIONALISED NITROSO COMPOUNDS A Thesis Presented by Sandra Luengo Arratta In Partial Fulfilment of the Requirements for the Award of the Degree of DOCTOR OF PHILOSOPHY OF UNIVERSITY COLLEGE LONDON Christopher Ingold Laboratories Department of Chemistry University College London 20 Gordon Street London WC1H 0AJ July 2010 DECLARATION I Sandra Luengo Arratta, confirm that the work presented in this thesis is my own. Where work has been derived from other sources, I confirm that this has been indicated in the thesis. ABSTRACT This thesis concerns the generation of geminally functionalised nitroso compounds and their subsequent use in intramolecular ene reactions of types I and II, in order to generate hydroxylamine derivatives which can evolve to the corresponding nitrones. The product nitrones can then be trapped in the inter- or intramolecular mode by a variety of reactions, including 1,3-dipolar cycloadditions, thereby leading to diversity oriented synthesis. The first section comprises the chemistry of the nitroso group with a brief discussion of the current methods for their generation together with the scope and limitations of these methods for carrying out nitroso ene reactions, with different examples of its potential as a powerful synthetic method to generate target drugs. The second chapter describes the results of the research programme and opens with the development of methods for the generation of functionalised nitroso compounds from different precursors including oximes and nitro compounds, using a range of reactants and conditions. The application of these methods in intramolecular nitroso ene reactions is then discussed. Chapter three presents the conclusions which have been drawn from the work presented in chapter two, and provides suggestions for possible directions of this research in the future. -

Download (6MB)
McLean, David (1980) Nitrosocarbonyl compounds in synthesis. PhD thesis http://theses.gla.ac.uk/4058/ Copyright and moral rights for this thesis are retained by the author A copy can be downloaded for personal non-commercial research or study, without prior permission or charge This thesis cannot be reproduced or quoted extensively from without first obtaining permission in writing from the Author The content must not be changed in any way or sold commercially in any format or medium without the formal permission of the Author When referring to this work, full bibliographic details including the author, title, awarding institution and date of the thesis must be given Glasgow Theses Service http://theses.gla.ac.uk/ [email protected] NITROSOCARDONY1COMPOUNDS IN SYNTHESIS A thesis presented in part fulfilment of the requirements for the Degree of Doctor of Philosophy by David McLean Department of Organic Chemistry University of Glasgow October, 1980. ACKNOWLEDGMENTS. I would like to take this opportunity to thank Professor Gordon W. Kirby for his friendly advice and discussions during the course of this work and in the preparation of this thesis. I also wish to thank my mum and dad, Maria Pita, Helen Prentice, Elizabeth Breslin and Dr. R. A. Hill. Finally I would like to express my thanks to the Science Research Council for financial assistance and the Staff of the Chemistry Department for technical assistance. CONTENTS. PAGE Smeary ..................................................... 1 Chapter 1 The Chemistry of Electrophilic C-Nitroao-Compounds .............................. 2 Chapter 2 Synthetic Applications of 2,2,2-Trichloroethyl Nitroeofoiate 2.1 Formation and Reduction of Diene/2,2,2-Trichloroethyl Nitrosoformate Adducts ..................................... -

7 September 1992.Pdf
* TODAY: LUOERIT'Z WOMEN·0N WARPATt:t * SEAL SCANDAL * SUPER WEEKEND SPORT * Bringing Africa South Vol.3 No.S Mon~ay September 7 1992 Farm worker faced 'brutal·torture' for relationship with white woman ',, ' I . STAFF REPORTER AS 'punishment' for daring to cross the colour lines and have a r~ation s hip with a white woman, a black farm labourer near Grootfontein was ailegedly subjected to 13 hours of torture, brutal assault and fQl'ced labour at the end of last week. According to one report, to dig trenches and plant fann wOlker Peter Aukumeb trees while assaulting him. (1 9) underwent his horrify ~pectorBrink~T~eb ing experience after two poiice station yesterday brothers found him . in the confinned the nature of the bedroom of their sister, allegations and said a charge Channaine Ras (20), on their of 'assault with intent to fann Shamalindi just north cause gri~vous bodily harm,' of Grootfontein last Thurs had been laid against the day night. two brothers while Aukumeb The two brothers, Schalk faces a charge of 'pointing and Juri Ras, are accused of a dangerous firearm '. torturing Aukumeb with an Aukumebwas apparently electronic cattle prodder; released from his ol'deal by tying his ankleS together with the police early on Friday horse ropes, and severely afternoon. beating him. In addition, According to a source in Aukumeb apparently claims the Ras brothers forced him To page 2 nTI""'l'T1IIo.Tr"' EXPERIENCE ... Anja SchrOder (centre) was crowned Miss Namibia at a gala function in Windhoek on Saturday night. Lindl! Schultz (left) was voted First princess, with Yolande Tait (right) Second Princess. -

Constitutional Court of South Africa
CONSTITUTIONAL COURT OF SOUTH AFRICA Case CCT 30/03 THE STATE versus BASSON Heard on : 21 – 25 February 2005 Decided on : 9 September 2005 JUDGMENT INDEX INTRODUCTION para 1 Background to the three issues raised in this Court para 3 (a) Bias para 3 (b) The admissibility of the bail record para 6 (c) The quashing of the charges para 13 I BIAS OF THE TRIAL JUDGE para 19 (a) Bias in February 2000 or at the end of the day? para 20 (b) The legal test for bias para 23 (c) Alleged specific manifestations of bias para 38 (i) Remarks and interventions by the judge para 41 (aa) The state was conducting “trial by ambush” para 45 (bb) The judge was “bored” by the state’s evidence para 46 (cc) Counsel for the state was “confused” para 48 (dd) The comment concerning state counsel’s ego para 49 (ee) Laughter about Asset Forfeiture application para 50 (ff) Judge’s comments concerning General Knobel’s evidence para 52 THE COURT (gg) Comment concerning witness’s sympathy for the accused para 55 (hh) Remarks concerning “Project Coast” para 58 (ii) Judge’s conduct during cross-examination of Dr Basson para 65 (jj) Assessment of these challenges para 66 (ii) Mistaken legal rulings and findings of fact para 69 (aa) Attorney-client privilege para 72 (bb) Refusal to call three further witnesses para 74 (cc) Implausibility of Dr Basson’s evidence para 80 (dd) Judge’s refusal to call another witness para 85 (ee) Erroneous factual finding: Mrs Webster para 87 (ff) Judge’s assessment of evidence: Dr Basson and General Knobel para 89 (gg) Erroneous factual finding: -

CONSTITUTIONAL COURT of SOUTH AFRICA Case CCT
CONSTITUTIONAL COURT OF SOUTH AFRICA Case CCT 30/03 THE STATE Applicant versus WOUTER BASSON Respondent Heard on : 4 and 5 November 2003 Decided on : 10 March 2004 JUDGMENT ACKERMANN J, MADALA J, MOKGORO J, MOSENEKE J, NGCOBO J, and O’REGAN J: [1] The state has applied to this Court for special leave to appeal against a judgment of the Supreme Court of Appeal (the SCA) in terms of rule 20 and, simultaneously, for leave to appeal directly to this Court against a judgment of the High Court in Pretoria (the High Court) in terms of rule 18. The respondent, Dr Wouter Basson, opposes both applications. Background ACKERMANN J et al [2] During 1999, the respondent, an employee of the South African National Defence Force, was charged in the High Court on 67 counts including murder, fraud, conspiracy to commit various crimes and drug offences. All the offences were allegedly committed before 1994 when the respondent worked in a division of the Defence Force called the Civil Co-operation Bureau. [3] During 1997 the accused was arrested, first on charges of contravening the Medicines and Related Substances Control Act, 101 of 1965, and later in the same year on charges of fraud. In relation to both sets of charges, bail hearings were held and the accused was granted bail. In relation to the fraud charges, the bail hearing was held during October and November 1997. The trial on all 67 charges (which now included charges of murder and conspiracy to commit various offences) commenced on 4 October 1999 before Hartzenberg J. -
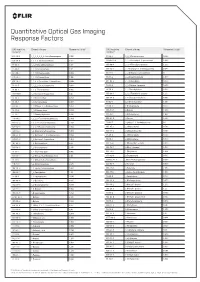
Quantitative Optical Gas Imaging Response Factors
Quantitative Optical Gas Imaging Response Factors CAS registry Chemical name Response factor† CAS registry Chemical name Response factor† number* number* 431-89-0 1_1_1_2_3_3_3-Heptafluoropropane 0.103 75-89-8 2_2_2-Trifluoroethanol 0.326 630-20-6 1_1_1_2-Tetrachloroethane 0.132 25256-77-4 2_2_4-Trimethyl-1_3-pentanediol 1.086 75-68-3 1_1_1-Chlorodifluoroethane 0.117 107-40-4 2_2_4-Trimethyl-2-pentene 1.262 71-55-6 1_1_1-Trichloroethane 0.121 306-83-2 2_2-Dichloro-1_1_1-trifluoroethane 0.047 421-50-1 1_1_1-Trifluoroacetone 0.086 76-11-9 2_2-Difluorotetrachloroethane 0 420-46-2 1_1_1-Trifluoroethane 0.106 75-83-2 2_2-Dimethyl_butane 1.944 354-14-3 1_1_2_2-Tetrachloro-1-fluoroethane 0.046 431-03-8 2_3-Butanedione 0.274 79-34-5 1_1_2_2-Tetrachloroethane 0.069 78-88-6 2_3-Dichloro-1-propene 0.279 79-00-5 1_1_2-Trichloroethane 0.102 79-29-8 2_3-Dimethylbutane 1.014 1717-00-6 1_1-Dichloro-1-fluoroethane 0.12 107-39-1 2_4_4-Trimethyl-1-pentene 1.212 75-34-3 1_1-Dichloroethane 0.272 584-84-9 2_4-Diisocyanatetoluene 0.895 75-35-4 1_1-Dichloroethene 0.017 87-62-7 2_6-Dimethylaniline 1.308 471-43-2 1_1-Difluoro-2_2-dichloroethane 0.352 75-26-3 2-Bromopropane 0.733 73-37-6 1_1-Difluoroethane 0.535 107-01-7 2-Butene 1.204 57-14-7 1_1-Dimethylhydrazine 0.897 111-76-2 2-Butoxyethanol 1.962 119-64-2 1_2_3_4-Tetrahydronaphthalene 2.439 554-61-0 2-Carene 1.286 488-23-3 1_2_3_4-Tetramethylbenzene 1.484 75-88-7 2-Chloro-1_1_1-trifluoroethane 0.1 527-53-7 1_2_3_5-Tetramethylbenzene 1.402 107-07-3 2-Chloroethanol 0.593 124-73-2 1_2-Dibomotetrafluoroethane -

PROLIFERATION: THREAT and RESPONSE January 2001
PROLIFERATION: THREAT AND RESPONSE January 2001 Office of the Secretary of Defense NT OF E D M E T F R E A N P S E E D U N A I C T I E R D E S T A M AT E S O F Proliferation: Threat and Response January 2001 This document, along with other DoD materials related to nonproliferation and counterproliferation, may be found at http://www.defenselink.mil Message of the Secretary of Defense At the dawn of the 21st Century, the United States now faces what could be called a Superpower Paradox. Our unrivaled supremacy in the conventional military arena is prompting adversaries to seek unconventional, asymmetric means to strike what they perceive as our Achilles heel. At least 25 countries now possess — or are in the process of acquiring and developing — capabilities to inflict mass casualties and destruction: nuclear, biologi- cal and chemical (NBC) weapons or the means to deliver them. For example: I North Korea is building and selling long-range missiles, has chemical and biologi- cal warfare capabilities, and may have diverted fissile material for nuclear weap- onry. I Iran, with foreign assistance, is buying and developing longer-range missiles, already has chemical weapons, and is seeking nuclear and biological capabilities. I Iraq — which prior to the 1991 Gulf War had developed chemical and biological weapons and associated delivery means, and was close to a nuclear capability — may have reconstituted these efforts since the departure of UN inspectors from Iraq in late 1998. I Libya has chemical capabilities and is trying to buy long-range missiles. -

Supplementary Information Cocrystal Design by Network-Based Link Prediction
Electronic Supplementary Material (ESI) for CrystEngComm. This journal is © The Royal Society of Chemistry 2019 Supplementary Information Cocrystal design by network-based link prediction Jan-Joris Devogelaer, Sander J.T. Brugman, Hugo Meekes, Paul Tinnemans, Elias Vlieg, René de Gelder Table of Contents S1 Materials and syntheses page 1 S2 Single-crystal X-ray structure determinations, ORTEP plots and H-Bonding page 6 a) CCDC 1940959 = p1812d: 4,4’-bipyridine + resorcinol b) CCDC 1940950 = p1823c: 1,2-di(4-pyridyl)ethylene + 4-aminobenzoic acid c) CCDC 1940956 = p1823a: 1,2-di(4-pyridyl)ethylene + 4-aminobenzoic acid dihydrate d) CCDC 1940954 = p1907a: 1,2-di(4-pyridyl)ethylene + sebacic acid e) CCDC 1940949 = p1818a: 4,4’-bipyridine + suberic acid f) CCDC 1940953 = p1922b: 1,2-di(4-pyridyl)ethylene + oxalic acid g) CCDC 1940955 = p1914b: 1,2-di(4-pyridyl)ethylene + oxalic acid dihydrate i) CCDC 1940951 = p1908a: 1,2-bis(4-pyridyl)ethane + salicylic acid j) CCDC 1940958 = p1926b: 1,2-di(4-pyridyl)ethylene + 4-nitrobenzoic acid k) CCDC 1940952 = p1910a: 1,2-di(4-pyridyl)ethylene + phthalic acid l) CCDC 1940957 = p1913a: 1,2-di(4-pyridyl)ethylene + malonic acid S3 Top 100 predictions of the scoring method page 17 S4 Predefined lists of common solvents and gases page 21 S1 - Materials and syntheses We first present the materials in Table S1, after which we discuss the protocol used to obtain crystals suitable for single-crystal X-ray diffraction. Besides the two-component structures shown in Table 2, an additional cocrystal dihydrate (c) and salt dihydrate (g) were found. -

'Sailor' Malan
Adolf ‘Sailor’ Malan Human Rights Activist & War Hero Adolf Gysbert ‘Sailor’ Malan was a leader of the largely white South African protest movement in the first few years of the 1950s known as the ‘Torch Commando’. The group was formed by the Springbok Legion and elements within the United Party through forming a non-partisan committee called the War Veterans Action Committee (WVAC). The agreement between the left leaders of the Springbok Legion, Cecil Williams and Jack Hodgson, and the United Party, was brokered by a Springbok Legion man in the United Party, Vic Clapham. These initiators agreed to put together a WVAC team made up of Adolf ‘Sailor’ Malan as president, Louis Kane- Berman, Ralph Parrott, Major Pretorius and Doreen Dunning. They were chosen because of their leadership role as soldiers, and because they professed non-partisanship in political party politics. This was not entirely true as some did have a role in the United Party, but this was outweighed by their military credentials. Springbok Legion personalities included many United Party members, such as Harry Schwartz who continued to play a major background organisational role in the Torch Commando. For his leadership role and for the things that he publicly said in defence of democracy, constitutionalism, justice, anti-racism and standing up for the poor and people of colour in particular, Sailor Malan was isolated and purged from historical memory. He was a Battle of Britain spitfire fighter pilot and war hero. As he was strongly anti- Nazi, he was dismayed at seeing it on the rise in his homeland. -

Enthalpies of Vaporization of Organic and Organometallic Compounds, 1880–2002
Enthalpies of Vaporization of Organic and Organometallic Compounds, 1880–2002 James S. Chickosa… Department of Chemistry, University of Missouri-St. Louis, St. Louis, Missouri 63121 William E. Acree, Jr.b… Department of Chemistry, University of North Texas, Denton, Texas 76203 ͑Received 17 June 2002; accepted 17 October 2002; published 21 April 2003͒ A compendium of vaporization enthalpies published within the period 1910–2002 is reported. A brief review of temperature adjustments of vaporization enthalpies from temperature of measurement to the standard reference temperature, 298.15 K, is included as are recently suggested reference materials. Vaporization enthalpies are included for organic, organo-metallic, and a few inorganic compounds. This compendium is the third in a series focusing on phase change enthalpies. Previous compendia focused on fusion and sublimation enthalpies. Sufficient data are presently available for many compounds that thermodynamic cycles can be constructed to evaluate the reliability of the measure- ments. A protocol for doing so is described. © 2003 American Institute of Physics. ͓DOI: 10.1063/1.1529214͔ Key words: compendium; enthalpies of condensation; evaporation; organic compounds; vaporization enthalpy. Contents inorganic compounds, 1880–2002. ............. 820 1. Introduction................................ 519 8. References to Tables 6 and 7.................. 853 2. Reference Materials for Vaporization Enthalpy Measurements.............................. 520 List of Figures 3. Heat Capacity Adjustments. ................. 520 1. A thermodynamic cycle for adjusting vaporization ϭ 4. Group Additivity Values for C (298.15 K) enthalpies to T 298.15 K.................... 521 pl 2. A hypothetical molecule illustrating the different Estimations................................ 521 hydrocarbon groups in estimating C ........... 523 5. A Thermochemical Cycle: Sublimation, p Vaporization, and Fusion Enthalpies...........