Elucidation of Molecular Mechanism of a Selective PPAR Modulator
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ezetimibe: a Novel Selective Cholesterol Absorption Inhibitor by Michele Koder, Pharm.D
OREGON DUR BOARD NEWSLETTER A N E VIDENCE B ASED D RUG T HERAPY R ESOURCE COPYRIGHT 2003 OREGON STATE UNIVERSITY. ALL RIGHTS RESERVED Volume 5, Issue 2 Also available on the web and via e-mail list-serve at February 2003 http://pharmacy.orst.edu/drug_policy/newsletter_email.html Ezetimibe: A novel selective cholesterol absorption inhibitor By Michele Koder, Pharm.D. , OSU College of Pharmacy Ezetimibe (Zetia) is a novel selective cholesterol absorption inhibitor that was approved by the FDA in October 2002. Unlike statins (HMG-CoA reductase inhibitors) and bile acid sequestrants, ezetimibe does not inhibit hepatic cholesterol synthesis or increase bile acid secretion. In contrast, ezetimibe selectively inhibits the uptake of dietary cholesterol from enterocytes in the brush border of the intestinal lumen resulting in a decrease in the delivery of dietary cholesterol to the liver and a subsequent decrease in hepatic cholesterol stores and increased cholesterol clearance from the blood.1 Ezetimibe’s unique action has generated interest in its use in combination with other cholesterol-lowering agents. It is indicated for the treatment of primary hypercholesterolemia as monotherapy and in combination with a statin. Ezetimibe is also approved for homozygous familial hypercholesterolemia and homozygous sitosterolemia. TABLE 1: EZETIMIBE CLINICAL TRIAL SUMMARY Study / Design Population Treatment % Change LDL % Change HDL % Change TG Bays et al3 N=432 EZ 5 mg -15.7 +2.9 MC, R, DB, PC LDL 130-250mg/dl EZ 10 mg -18.5 +3.5 NS 12 wk; Phase II TG -

The Antidiabetic Drug Lobeglitazone Has the Potential to Inhibit PTP1B T Activity ⁎ Ruth F
Bioorganic Chemistry 100 (2020) 103927 Contents lists available at ScienceDirect Bioorganic Chemistry journal homepage: www.elsevier.com/locate/bioorg The antidiabetic drug lobeglitazone has the potential to inhibit PTP1B T activity ⁎ Ruth F. Rochaa, Tiago Rodriguesc, Angela C.O. Menegattia,b, , Gonçalo J.L. Bernardesc,d, Hernán Terenzia a Centro de Biologia Molecular Estrutural, Departamento de Bioquímica, Universidade Federal de Santa Catarina, Campus Trindade, 88040-900 Florianópolis, SC, Brazil b Universidade Federal do Piauí, CPCE, 64900-000 Bom Jesus, PI, Brazil c Instituto de Medicina Molecular, Faculdade de Medicina, Universidade de Lisboa, Avenida Professor Egas Moniz, 1649-028 Lisbon, Portugal d Department of Chemistry, University of Cambridge, Lensfield Road, CB2 1EW Cambridge, UK ARTICLE INFO ABSTRACT Keywords: Protein tyrosine phosphatase 1B (PTP1B) is considered a potential therapeutic target for the treatment of type 2 Thiazolidinediones diabetes mellitus (T2DM), since this enzyme plays a significant role to down-regulate insulin and leptin sig- Lobeglitazone nalling and its over expression has been implicated in the development of insulin resistance, T2DM and obesity. PPAR-γ Some thiazolidinediones (TZD) derivatives have been reported as promising PTP1B inhibitors with anti hy- PTP1B perglycemic effects. Recently, lobeglitazone, a new TZD, was described as an antidiabetic drug that targetsthe Non-competitive inhibitors PPAR-γ (peroxisome γ proliferator-activated receptor) pathway, but no information on its effects on PTP1B have been reported to date. We investigated the effects of lobeglitazone on PTP1B activity in vitro. Surprisingly, lobeglitazone led to moderate inhibition on PTP1B (IC50 42.8 ± 3.8 µM) activity and to a non-competitive reversible mechanism of action. -
Statins and Lipid Lowering Agents
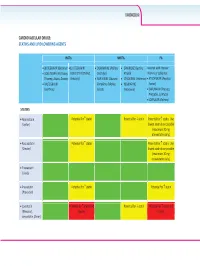
CARDIOVASCULAR Z/Ks^h>ZZh'^͗ ^dd/E^E>/W/>KtZ/E''Ed^ /E^d/Ɛ EEZd/Ɛ W/Ɛ x/d'Zs/Z ;ŝŬƚĂƌǀLJͿ x>s/d'Zs/Zͬ x KZs/Z/E ;WŝĨĞůƚƌŽ͕ x &s/ZE ;^ƵƐƚŝǀĂ͕ ŽŽƐƚĞĚǁŝƚŚƌŝƚŽŶĂǀŝƌ xK>hd'Zs/Z ;dŝǀŝĐĂLJ͕ K//^dd ;^ƚƌŝďŝůĚ͕ ĞůƐƚƌŝŐŽͿ ƚƌŝƉůĂͿ ;EŽƌǀŝƌͿŽƌĐŽďŝĐŝƐƚĂƚ dƌŝƵŵĞƋ͕:ƵůƵĐĂ͕ŽǀĂƚŽͿ 'ĞŶǀŽLJĂͿ x Z/>W/s/Z/E ;ĚƵƌĂŶƚ͕ x dZs/Z/E ;/ŶƚĞůĞŶĐĞͿ xdEs/Z ;ZĞLJĂƚĂnj͕ xZ>d'Zs/Z ŽŵƉůĞƌĂ͕KĚĞĨƐĞLJ͕ x Es/ZW/E ǀŽƚĂnjͿ ;/ƐĞŶƚƌĞƐƐͿ :ƵůƵĐĂͿ ;sŝƌĂŵƵŶĞͿ xZhEs/Z ;WƌĞnjŝƐƚĂ͕ WƌĞnjĐŽďŝdž͕^LJŵƚƵnjĂͿ x>KW/Es/Z ;<ĂůĞƚƌĂͿ ^dd/E^ xƚŽƌǀĂƐƚĂƚŝŶ WŽƚĞŶƚŝĂůĨŽƌ nƐƚĂƚŝŶ WŽƚĞŶƚŝĂůĨŽƌ pƐƚĂƚŝŶ WŽƚĞŶƚŝĂůĨŽƌ nƐƚĂƚŝŶ͘hƐĞ ;>ŝƉŝƚŽƌͿ ůŽǁĞƐƚƐƚĂƚŝŶĚŽƐĞƉŽƐƐŝďůĞ ;ŵĂdžŝŵƵŵϮϬŵŐ ĂƚŽƌǀĂƐƚĂƚŝŶĚĂŝůLJͿ͘ xZŽƐƵǀĂƐƚĂƚŝŶ WŽƚĞŶƚŝĂůĨŽƌ nƐƚĂƚŝŶ WŽƚĞŶƚŝĂůĨŽƌ nƐƚĂƚŝŶ͘hƐĞ ;ƌĞƐƚŽƌͿ ůŽǁĞƐƚƐƚĂƚŝŶĚŽƐĞƉŽƐƐŝďůĞ ;ŵĂdžŝŵƵŵϭϬŵŐ ƌŽƐƵǀĂƐƚĂƚŝŶĚĂŝůLJͿ͘ xWŝƚĂǀĂƐƚĂƚŝŶ ;>ŝǀĂůŽͿ xWƌĂǀĂƐƚĂƚŝŶ WŽƚĞŶƚŝĂůĨŽƌ nƐƚĂƚŝŶ WŽƚĞŶƚŝĂůĨŽƌ nƐƚĂƚŝŶ ;WƌĂǀĂĐŚŽůͿ x>ŽǀĂƐƚĂƚŝŶ WŽƚĞŶƚŝĂůĨŽƌ nƐƚĂƚŝŶĂŶĚ WŽƚĞŶƚŝĂůĨŽƌ pƐƚĂƚŝŶ WŽƚĞŶƚŝĂůĨŽƌ nƐƚĂƚŝŶĂŶĚ ;DĞǀĂĐŽƌͿ͕ ƚŽdžŝĐŝƚLJ ƚŽdžŝĐŝƚLJ ƐŝŵǀĂƐƚĂƚŝŶ ;ŽĐŽƌͿ CARDIOVASCULAR INSTIs NNRTIs PIs xBICTEGRAVIR (Biktarvy) xELVITEGRAVIR/ x DORAVIRINE (Pifeltro, x EFAVIRENZ (Sustiva, Boosted with ritonavir xDOLUTEGRAVIR (Tivicay, COBICISTAT (Stribild, Delstrigo) Atripla) (Norvir) or cobicistat Triumeq, Juluca) Genvoya) x RILPIVIRINE (Edurant, x ETRAVIRINE (Intelence) xATAZANAVIR (Reyataz, xRALTEGRAVIR Complera, Odefsey, x NEVIRAPINE Evotaz) (Isentress) Juluca) (Viramune) xDARUNAVIR (Prezista, Prezcobix, Symtuza) xLOPINAVIR (Kaletra) FIBRATES xFenofibrate, bezafibrate, gemfibrozil CHOLESTEROL ABSORPTION INHIBITOR xEzetimibe -

FIELD Study Revealed Fenofibrate Reduced Need for Laser Treatment for Diabetic Retinopathy by Anthony C
Supplement to Supported by an unrestricted educational grant from Abbott Laboratories March/April 2008 FIELD Study Revealed Fenofibrate Reduced Need for Laser Treatment for Diabetic Retinopathy By Anthony C. Keech, MBBS, Msc Epid, FRANZCS, FRACP; and Paul Mitchell, MBBS(Hons), MD, PhD, FRANZCO, FRACS, FRCOphth, FAFPHM This agent’s mechanism of benefit in diabetic retinopathy appears to go beyond its effects on lipid concentration or blood pressure, and this potential mechanism of action operates even when glycemic control and blood pressure levels are within goal. ABSTRACT icant relative reduction was seen of almost one-third in PURPOSE the rate of first laser application for retinopathy after The FIELD (Fenofibrate Intervention and Event an average treatment duration of 5 years with fenofi- Lowering in Diabetes) study sought to investigate brate 200 mg/day. whether long-term lipid-lowering therapy with fenofi- In this report, we detail the effects of fenofibrate brate would reduce macro- and microvascular compli- administration on ophthalmic microvascular compli- cations among patients with type 2 diabetes. We previ- cations and attempt to clarify some of the underlying ously reported that in type 2 diabetes patients with pathologies being addressed among patients undergo- adequate glycemic and blood pressure control, a signif- ing laser treatment. Jointly sponsored by The Dulaney Foundation and Retina Today MARCH/APRIL 2008 I SUPPLEMENT TO RETINA TODAY I 1 FIELD Study Revealed Fenofibrate Reduced Need for Laser Treatment for Diabetic Retinopathy Jointly sponsored by The Dulaney Foundation and Retina Today. Release date: April 2008. Expiration date: April 2009. This continuing medical education activity is supported by an unrestricted educational grant from Abbott Laboratories. -

Classification Decisions Taken by the Harmonized System Committee from the 47Th to 60Th Sessions (2011
CLASSIFICATION DECISIONS TAKEN BY THE HARMONIZED SYSTEM COMMITTEE FROM THE 47TH TO 60TH SESSIONS (2011 - 2018) WORLD CUSTOMS ORGANIZATION Rue du Marché 30 B-1210 Brussels Belgium November 2011 Copyright © 2011 World Customs Organization. All rights reserved. Requests and inquiries concerning translation, reproduction and adaptation rights should be addressed to [email protected]. D/2011/0448/25 The following list contains the classification decisions (other than those subject to a reservation) taken by the Harmonized System Committee ( 47th Session – March 2011) on specific products, together with their related Harmonized System code numbers and, in certain cases, the classification rationale. Advice Parties seeking to import or export merchandise covered by a decision are advised to verify the implementation of the decision by the importing or exporting country, as the case may be. HS codes Classification No Product description Classification considered rationale 1. Preparation, in the form of a powder, consisting of 92 % sugar, 6 % 2106.90 GRIs 1 and 6 black currant powder, anticaking agent, citric acid and black currant flavouring, put up for retail sale in 32-gram sachets, intended to be consumed as a beverage after mixing with hot water. 2. Vanutide cridificar (INN List 100). 3002.20 3. Certain INN products. Chapters 28, 29 (See “INN List 101” at the end of this publication.) and 30 4. Certain INN products. Chapters 13, 29 (See “INN List 102” at the end of this publication.) and 30 5. Certain INN products. Chapters 28, 29, (See “INN List 103” at the end of this publication.) 30, 35 and 39 6. Re-classification of INN products. -

Clinofibrate Improved Canine Lipid Metabolism in Some but Not All Breeds
NOTE Internal Medicine Clinofibrate improved canine lipid metabolism in some but not all breeds Yohtaro SATO1), Nobuaki ARAI2), Hidemi YASUDA3) and Yasushi MIZOGUCHI4)* 1)Graduate School of Agriculture, Meiji University, 1-1-1 Higashimita, Tama-ku, Kawasaki, Kanagawa 214-8571, Japan 2)Spectrum Lab Japan, 1-5-22-201 Midorigaoka, Meguro-ku, Tokyo 152-0034, Japan 3)Yasuda Veterinary Clinic, 1-5-22 Midorigaoka, Meguro-ku, Tokyo 152-0034, Japan 4)School of Agriculture, Meiji University, 1-1-1 Higashimita, Tama-ku, Kawasaki, Kanagawa 214-8571, Japan ABSTRACT. The objectives of this study were to assess if Clinofibrate (CF) treatment improved J. Vet. Med. Sci. lipid metabolism in dogs, and to clarify whether its efficacy is influenced by canine characteristics. 80(6): 945–949, 2018 We collected medical records of 306 dogs and performed epidemiological analyses. Lipid values of all lipoproteins were significantly decreased by CF medication, especially VLDL triglyceride doi: 10.1292/jvms.17-0703 (TG) concentration (mean reduction rate=54.82%). However, 17.65% of dogs showed drug refractoriness in relation to TG level, and Toy Poodles had a lower CF response than other breeds (OR=5.36, 95% CI=2.07–13.90). Therefore, our study suggests that genetic factors may have an Received: 22 December 2017 effect on CF response, so genetic studies on lipid metabolism-related genes might be conducted Accepted: 9 March 2018 to identify variations in CF efficacy. Published online in J-STAGE: KEY WORDS: clinofibrate, descriptive epidemiology, drug response, dyslipidemia, Toy Poodle 26 March 2018 High serum cholesterol (Cho) and triglyceride (TG) concentrations in dogs are caused by various factors such as lack of exercise, high fat diets, obesity, neutralization, age, diseases and breed [6, 21, 24]. -

AVANDIA (Rosiglitazone Maleate Tablets), for Oral Use Ischemic Cardiovascular (CV) Events Relative to Placebo, Not Confirmed in Initial U.S
HIGHLIGHTS OF PRESCRIBING INFORMATION ----------------------- WARNINGS AND PRECAUTIONS ----------------------- These highlights do not include all the information needed to use • Fluid retention, which may exacerbate or lead to heart failure, may occur. AVANDIA safely and effectively. See full prescribing information for Combination use with insulin and use in congestive heart failure NYHA AVANDIA. Class I and II may increase risk of other cardiovascular effects. (5.1) • Meta-analysis of 52 mostly short-term trials suggested a potential risk of AVANDIA (rosiglitazone maleate tablets), for oral use ischemic cardiovascular (CV) events relative to placebo, not confirmed in Initial U.S. Approval: 1999 a long-term CV outcome trial versus metformin or sulfonylurea. (5.2) • Dose-related edema (5.3) and weight gain (5.4) may occur. WARNING: CONGESTIVE HEART FAILURE • Measure liver enzymes prior to initiation and periodically thereafter. Do See full prescribing information for complete boxed warning. not initiate therapy in patients with increased baseline liver enzyme levels ● Thiazolidinediones, including rosiglitazone, cause or exacerbate (ALT >2.5X upper limit of normal). Discontinue therapy if ALT levels congestive heart failure in some patients (5.1). After initiation of remain >3X the upper limit of normal or if jaundice is observed. (5.5) AVANDIA, and after dose increases, observe patients carefully for signs • Macular edema has been reported. (5.6) and symptoms of heart failure (including excessive, rapid weight gain; • Increased incidence of bone fracture was observed in long-term trials. dyspnea; and/or edema). If these signs and symptoms develop, the heart (5.7) failure should be managed according to current standards of care. -

The Effect of Rosiglitazone on Overweight Subjects with Type 1 Diabetes
Clinical Care/Education/Nutrition ORIGINAL ARTICLE The Effect of Rosiglitazone on Overweight Subjects With Type 1 Diabetes SUZANNE M. STROWIG, MSN, RN the potentially serious consequences of PHILIP RASKIN, MD excessive weight gain and insulin- induced hypoglycemia, investigators con- tinue to search for treatments that address both the treatment of insulin deficiency as well as other metabolic abnormalities that OBJECTIVE — To evaluate the safety and effectiveness of rosiglitazone in the treatment of are associated with diabetes (5). overweight subjects with type 1 diabetes. Insulin resistance, a metabolic abnor- mality common in type 2 diabetes, ap- RESEARCH DESIGN AND METHODS — A total of 50 adult type 1 diabetic subjects 2 pears to be present in individuals with with a baseline BMI Ն27 kg/m were randomly assigned in a double-blind fashion to take insulin and placebo (n ϭ 25) or insulin and rosiglitazone 4 mg twice daily (n ϭ 25) for a period of 8 type 1 diabetes, as well. Although insulin months. Insulin regimen and dosage were modified in all subjects to achieve near-normal resistance in type 2 diabetes is generally glycemic control. associated with obesity, hypertension, dyslipidemia, and other metabolic disor- RESULTS — Both groups experienced a significant reduction in HbA1c (A1C) level (rosigli- ders, studies have shown that overweight tazone: 7.9 Ϯ 1.3 to 6.9 Ϯ 0.7%, P Ͻ 0.0001; placebo: 7.7 Ϯ 0.8 to 7.0 Ϯ 0.9%, P ϭ 0.002) and as well as normal-weight adults with type a significant increase in weight (rosiglitazone: 97.2 Ϯ 11.8 to 100.6 Ϯ 16.0 kg, P ϭ 0.008; 1 diabetes can have peripheral and he- Ϯ Ϯ ϭ ϭ placebo: 96.4 12.2 to 99.1 15.0, P 0.016). -

Effects of Clofibrate Derivatives on Hyperlipidemia Induced by a Cholesterol-Free, High-Fructose Diet in Rats
Showa Univ. J. Med. Sci. 7(2), 173•`182, December 1995 Original Effects of Clofibrate Derivatives on Hyperlipidemia Induced by a Cholesterol-Free, High-Fructose Diet in Rats Hideyukl KURISHIMA,Sadao NAKAYAMA,Minoru FURUYA and Katsuji OGUCHI Abstract: The effects of the clofibrate derivatives fenofibrate (FF), bezafibrate (BF), and clinofibrate (CF), on hyperlipidemia induced by a cholesterol-free, high-fructose diet (HFD) in rats were investigated. Feeding of HFD for 2 weeks increased the high-density lipoprotein subfraction (HDL1) and decreased the low-density lipoprotein (LDL) fraction. The levels of total cholesterol (TC), free cholesterol, triglyceride (TG), and phospholipid in serum were increased by HFD feeding. Administration of CF inhibited the increase in HDL1 content. All three agents inhibited the decrease in LDL level. Both BF and CF decreased VLDL level. Administration of FF, BF, or CF inhibited the increases of serum lipids, especially that of TC and TG. The inhibitory effects of CF on HFD- induced increases in HDL1, TC, and TG were greater than those of FF and BF. These results demonstrate that FF, BF, and CF improve the intrinsic hyper- lipidemia induced by HFD feeding in rats. Key words: fenofibrate, bezafibrate, clinofibrate, fructose-induced hyperlipide- mia, lipoprotein. Introduction Clofibrate is one of the most effective antihypertriglycedemic agents currently available. However, because of its adverse effects, such as hepatomegaly1, several derivatives, such as clinofibrate (CF) and bezafibrate (BF) have been developed which are more effective and have fewer adverse effects. For example, it has been shown that the hypolipidemic effect of CF is greater than that of clofibrate while its tendency to produce hepatomegaly is less1. -

Pemafibrate, a Novel Selective Peroxisome Proliferator-Activated
www.nature.com/scientificreports OPEN Pemafibrate, a novel selective peroxisome proliferator-activated receptor alpha modulator, Received: 25 July 2016 Accepted: 11 January 2017 improves the pathogenesis in Published: 14 February 2017 a rodent model of nonalcoholic steatohepatitis Yasushi Honda1, Takaomi Kessoku1, Yuji Ogawa1, Wataru Tomeno1, Kento Imajo1, Koji Fujita1, Masato Yoneda1, Toshiaki Takizawa2, Satoru Saito1, Yoji Nagashima3 & Atsushi Nakajima1 The efficacy of peroxisome proliferator-activated receptorα -agonists (e.g., fibrates) against nonalcoholic fatty liver disease (NAFLD)/nonalcoholic steatohepatitis (NASH) in humans is not known. Pemafibrate is a novel selective peroxisome proliferator-activated receptorα modulator that can maximize the beneficial effects and minimize the adverse effects of fibrates used currently. In a phase-2 study, pemafibrate was shown to improve liver dysfunction in patients with dyslipidaemia. In the present study, we first investigated the effect of pemafibrate on rodent models of NASH. Pemafibrate efficacy was assessed in a diet-induced rodent model of NASH compared with fenofibrate. Pemafibrate and fenofibrate improved obesity, dyslipidaemia, liver dysfunction, and the pathological condition of NASH. Pemafibrate improved insulin resistance and increased energy expenditure significantly. To investigate the effects of pemafibrate, we analysed the gene expressions and protein levels involved in lipid metabolism. We also analysed uncoupling protein 3 (UCP3) expression. Pemafibrate stimulated lipid turnover and upregulated UCP3 expression in the liver. Levels of acyl-CoA oxidase 1 and UCP3 protein were increased by pemafibrate significantly. Pemafibrate can improve the pathogenesis of NASH by modulation of lipid turnover and energy metabolism in the liver. Pemafibrate is a promising therapeutic agent for NAFLD/NASH. The incidence of nonalcoholic fatty liver disease (NAFLD) is increasing worldwide. -

AVANDIA® (Rosiglitazone Maleate) Tablets
PRESCRIBING INFORMATION AVANDIA® (rosiglitazone maleate) Tablets WARNING: CONGESTIVE HEART FAILURE ● Thiazolidinediones, including rosiglitazone, cause or exacerbate congestive heart failure in some patients (see WARNINGS). After initiation of AVANDIA, and after dose increases, observe patients carefully for signs and symptoms of heart failure (including excessive, rapid weight gain, dyspnea, and/or edema). If these signs and symptoms develop, the heart failure should be managed according to current standards of care. Furthermore, discontinuation or dose reduction of AVANDIA must be considered. ● AVANDIA is not recommended in patients with symptomatic heart failure. Initiation of AVANDIA in patients with established NYHA Class III or IV heart failure is contraindicated. (See CONTRAINDICATIONS and WARNINGS.) DESCRIPTION AVANDIA (rosiglitazone maleate) is an oral antidiabetic agent which acts primarily by increasing insulin sensitivity. AVANDIA is used in the management of type 2 diabetes mellitus (also known as non-insulin-dependent diabetes mellitus [NIDDM] or adult-onset diabetes). AVANDIA improves glycemic control while reducing circulating insulin levels. Pharmacological studies in animal models indicate that rosiglitazone improves sensitivity to insulin in muscle and adipose tissue and inhibits hepatic gluconeogenesis. Rosiglitazone maleate is not chemically or functionally related to the sulfonylureas, the biguanides, or the alpha-glucosidase inhibitors. Chemically, rosiglitazone maleate is (±)-5-[[4-[2-(methyl-2- pyridinylamino)ethoxy]phenyl]methyl]-2,4-thiazolidinedione, (Z)-2-butenedioate (1:1) with a molecular weight of 473.52 (357.44 free base). The molecule has a single chiral center and is present as a racemate. Due to rapid interconversion, the enantiomers are functionally indistinguishable. The structural formula of rosiglitazone maleate is: The molecular formula is C18H19N3O3S•C4H4O4. -

A61p1/16 (2006.01) A61p3/00 (2006.01) Km, Ml, Mr, Ne, Sn, Td, Tg)
( (51) International Patent Classification: TR), OAPI (BF, BJ, CF, CG, Cl, CM, GA, GN, GQ, GW, A61P1/16 (2006.01) A61P3/00 (2006.01) KM, ML, MR, NE, SN, TD, TG). A61K 31/192 (2006.01) C07C 321/28 (2006.01) Declarations under Rule 4.17: (21) International Application Number: — as to the applicant's entitlement to claim the priority of the PCT/IB2020/000808 earlier application (Rule 4.17(iii)) (22) International Filing Date: Published: 25 September 2020 (25.09.2020) — with international search report (Art. 21(3)) (25) Filing Language: English — before the expiration of the time limit for amending the claims and to be republished in the event of receipt of (26) Publication Language: English amendments (Rule 48.2(h)) (30) Priority Data: 62/906,288 26 September 2019 (26.09.2019) US (71) Applicant: ABIONYX PHARMA SA [FR/FR] ; 33-43 Av¬ enue Georges Pompidou, Batiment D, 31130 Bahna (FR). (72) Inventor: DASSEUX, Jean-Louis, Henri; 7 Allees Charles Malpel, Bat. B, 31300 Toulouse (FR). (74) Agent: HOFFMANN EITLE PATENT- UND RECHTSANWALTE PARTMBB, ASSOCIATION NO. 151; Arabellastrasse 30, 81925 Munich (DE). (81) Designated States (unless otherwise indicated, for every kind of national protection available) : AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DJ, DK, DM, DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, HR, HU, ID, IL, IN, IR, IS, IT, JO, JP, KE, KG, KH, KN, KP, KR, KW, KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, SD, SE, SG, SK, SL, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, WS, ZA, ZM, ZW.