Explaining Intraspecific Diversity in Plant Secondary Metabolites in An
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Intestinal Anti-Inflammatory Activity of Terpenes in Experimental Models
molecules Review Intestinal Anti-Inflammatory Activity of Terpenes in Experimental Models (2010–2020): A Review Maria Elaine Araruna 1, Catarina Serafim 1, Edvaldo Alves Júnior 1, Clelia Hiruma-Lima 2, Margareth Diniz 1,3 and Leônia Batista 1,3,* 1 Postgraduate Program in Natural Products and Bioactive Synthetic, Health Sciences Center, Federal University of Paraiba, João Pessoa 58051-900, PB, Brazil; [email protected] (M.E.A.); [email protected] (C.S.); [email protected] (E.A.J.); [email protected] (M.D.) 2 Department of Structural and Functional Biology (Physiology), Institute of Biosciences, São Paulo State University, Botucatu 18618-970, SP, Brazil; [email protected] 3 Department of Pharmacy, Health Sciences Center, Federal University of Paraíba, João Pessoa 58051-900, PB, Brazil * Correspondence: [email protected]; Tel.: +55-83-32167003; Fax: +55-83-32167502 Academic Editors: Maurizio Battino, Jesus Simal-Gandara and Esra Capanoglu Received: 8 September 2020; Accepted: 28 September 2020; Published: 20 November 2020 Abstract: Inflammatory bowel diseases (IBDs) refer to a group of disorders characterized by inflammation in the mucosa of the gastrointestinal tract, which mainly comprises Crohn’s disease (CD) and ulcerative colitis (UC). IBDs are characterized by inflammation of the intestinal mucosa, are highly debilitating, and are without a definitive cure. Their pathogenesis has not yet been fully elucidated; however, it is assumed that genetic, immunological, and environmental factors are involved. People affected by IBDs have relapses, and therapeutic regimens are not always able to keep symptoms in remission over the long term. Natural products emerge as an alternative for the development of new drugs; bioactive compounds are promising in the treatment of several disorders, among them those that affect the gastrointestinal tract, due to their wide structural diversity and biological activities. -

Bilirubin: an Animal Pigment in the Zingiberales and Diverse Angiosperm Orders Cary L
Florida International University FIU Digital Commons FIU Electronic Theses and Dissertations University Graduate School 11-5-2010 Bilirubin: an Animal Pigment in the Zingiberales and Diverse Angiosperm Orders Cary L. Pirone Florida International University, [email protected] DOI: 10.25148/etd.FI10122201 Follow this and additional works at: https://digitalcommons.fiu.edu/etd Part of the Biochemistry Commons, and the Botany Commons Recommended Citation Pirone, Cary L., "Bilirubin: an Animal Pigment in the Zingiberales and Diverse Angiosperm Orders" (2010). FIU Electronic Theses and Dissertations. 336. https://digitalcommons.fiu.edu/etd/336 This work is brought to you for free and open access by the University Graduate School at FIU Digital Commons. It has been accepted for inclusion in FIU Electronic Theses and Dissertations by an authorized administrator of FIU Digital Commons. For more information, please contact [email protected]. FLORIDA INTERNATIONAL UNIVERSITY Miami, Florida BILIRUBIN: AN ANIMAL PIGMENT IN THE ZINGIBERALES AND DIVERSE ANGIOSPERM ORDERS A dissertation submitted in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY in BIOLOGY by Cary Lunsford Pirone 2010 To: Dean Kenneth G. Furton College of Arts and Sciences This dissertation, written by Cary Lunsford Pirone, and entitled Bilirubin: An Animal Pigment in the Zingiberales and Diverse Angiosperm Orders, having been approved in respect to style and intellectual content, is referred to you for judgment. We have read this dissertation and recommend that it be approved. ______________________________________ Bradley C. Bennett ______________________________________ Timothy M. Collins ______________________________________ Maureen A. Donnelly ______________________________________ John. T. Landrum ______________________________________ J. Martin Quirke ______________________________________ David W. Lee, Major Professor Date of Defense: November 5, 2010 The dissertation of Cary Lunsford Pirone is approved. -

Thesis-1966D-R342b.Pdf
I I THE BIOSYNTHESIS OF NEPETALACTONE By FREDERICK EUGENE REGNIER 01 Bachelor of Science Peru State College Peru, Nebraska 1960 Submitted to the Faculty of the Graduate School of the Oklahoma State University in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY May, 1966 THE BIOSYNTHESIS OF NEPETALACTONE Thesis Approved: l/ ~;==chool ii ACKNOWLEDGEMENTS The author wishes to express his gratitude and appreciation to: Dr. G. R. Waller and Dr. E. J. Eisenbraun for their guidance, counsel and assistance during the course of this study. Dr. E. C. Horning for placing the combined mass spectrometer-gas chromatograph at my disposal. Mr. Sten Wikstrom for his technical assistance in operating the mass spectrometer. Dr. M. H. Brooks for her assistance in the histological studies. Dr. W. R. Kays for providing plant material. Dr. V. T. Waterfall for making botanical classification. Dr. H. Auda and Mr. G. V. Odell for their suggestions and technical assistance. Mrs. Linda M. Regnier for her assistance and encouragement during this study. iii TABLE OF CONTENTS Chapter Page I. INTRODUCTION 1 II. LITERATURE REVIEW 3 III. COMPOSITION OF THE ESSENTIAL OIL OF NEPETA cataria L.. 13 IV. THE DISTRIBUTION AND ANALYSIS OF NEPETALACTONE ISOMERS FROM DIFFERENT NEPETA SPECIES 54 V. THE BIOSYNTHESIS OF NEPETALACTONE 70 VI. SUMMARY 103 BIBLIOGRAPHY 105 iv LIST OF TABLES Table Page CHAPTER III I. Tabulation of the Intense Ions in the Spectra of the Compounds in Fraction A 21+ II. Tabulation of the Intense Ions in the Spectra of the Compounds in Fraction B 27 III. Tabulation of the Intense Ions in the Spectrum of Sample c4 . -

Nutritional and Medicinal Properties of Solanaceous Vegetables Shilpa Devi and Arvind Nagar Division of Vegetable Science, IARI, New Delhi, 110012
Shilpa Devi and Arvind Nagar Your full article ( between 500 to 5000 words) - - Do check for grammatical errors or spelling mistakes Nutritional and Medicinal Properties of Solanaceous Vegetables Shilpa Devi and Arvind Nagar Division of vegetable Science, IARI, New Delhi, 110012 Corresponding mail- [email protected] Introduction The family Solanaceae, or nightshades, is an economically important family of flowering plants. The family ranges from annual and perennial herbs to vines, or either shrubs, and trees, including a number of important vegetable crops like tomato, pepper, eggplant, white and red potato, and tomatillo. This family also contains several plants that are considered toxic to humans being such as the weeds jimsonweed, nightshade and mandrake. Many members of the family contain potent alkaloids that are having immense value by considering its nutritional value. The family belongs to the order Solanales, in the asterid group dicotyledons (Magnoliopsida). The solanaceae consists of approximately 98 genera and about 2,700 species, with a great diversity in their habitats, morphology and ecology. Worldwide 53% of children are malnourished and underweight with 40% of them living in India. Solanaceous vegetable crops are important source of vitamin C, A, E, thiamine, niacin, pyridoxine, folacin, minerals and dietry fibres which play a significant role in human nutrition and helps to cope with malnutrition. Nutritional and Medicinal Properties of Tomato Tomatoes are the 2nd highly produced and consumed vegetable in the world today. Tomato is consumed either fresh or in many processed forms like ketchup, canned whole or in pieces, puree, sauce, soup, juice, or sun- dried. Tomato fruits are considered a low energy dense food with unique constituents that may positively affect health. -

Effect of Prebiotic Supplementation on Performance and Health of Dairy Calves: Protocol for a Systematic Review and Meta-Analysis R
Effect of prebiotic supplementation on performance and health of dairy calves: protocol for a systematic review and meta-analysis R. B. Lopes1, E. D. Fausak1, C. Bernal-Córdoba1, N. Silva-del-Río1* 1University of California Davis, US; [email protected] (RBL); [email protected] (EDF); [email protected] (CBC); [email protected] (NSDR) *Corresponding author: Veterinary Medicine Teaching and Research Center, University of California Davis, 18830 Road 112, Tulare, CA, 93274, Phone: 559-688-1731, Fax: 559-686- 4231. Author Contributions: RBL: Assist to develop search strategy and literature search, data screening, extraction, statistical analysis, and protocol and manuscript preparation. EDF: Develop search strategy and literature search, edit protocol. CBC: Data screening, review data extraction, review protocol and manuscript. NSDR: Assist, edit and review protocol and manuscript. Amendments: Any amendments to this protocol will be documented and justified in the final review. Support: This study is financially supported by the California Department of Food and Agriculture (CDFA) and it is part of the Antimicrobial Use and Stewardship (AUS) program. INTRODUCTION Rationale In the dairy industry, the treatment of sick calves is commonly based on the antibiotic use by systematic therapy or addition into the milk (Foutz et al., 2018; Urie et al., 2018). The overuse and misuse of antibiotics contribute to the development of antimicrobial resistance, which is a threat to both animal and human health. Thus, it is fundamental to investigate preventative strategies, such as prebiotics, to reduce the incidence of diseases and consequently the use of antibiotics. According to the International Scientific Association for Probiotics and Prebiotics, prebiotic is a “substrate that is selectively utilized by host microorganisms conferring a health benefit” (Gibson et al., 2017). -

Natural Products (Secondary Metabolites)
Biochemistry & Molecular Biology of Plants, B. Buchanan, W. Gruissem, R. Jones, Eds. © 2000, American Society of Plant Physiologists CHAPTER 24 Natural Products (Secondary Metabolites) Rodney Croteau Toni M. Kutchan Norman G. Lewis CHAPTER OUTLINE Introduction Introduction Natural products have primary ecological functions. 24.1 Terpenoids 24.2 Synthesis of IPP Plants produce a vast and diverse assortment of organic compounds, 24.3 Prenyltransferase and terpene the great majority of which do not appear to participate directly in synthase reactions growth and development. These substances, traditionally referred to 24.4 Modification of terpenoid as secondary metabolites, often are differentially distributed among skeletons limited taxonomic groups within the plant kingdom. Their functions, 24.5 Toward transgenic terpenoid many of which remain unknown, are being elucidated with increas- production ing frequency. The primary metabolites, in contrast, such as phyto- 24.6 Alkaloids sterols, acyl lipids, nucleotides, amino acids, and organic acids, are 24.7 Alkaloid biosynthesis found in all plants and perform metabolic roles that are essential 24.8 Biotechnological application and usually evident. of alkaloid biosynthesis Although noted for the complexity of their chemical structures research and biosynthetic pathways, natural products have been widely per- 24.9 Phenylpropanoid and ceived as biologically insignificant and have historically received lit- phenylpropanoid-acetate tle attention from most plant biologists. Organic chemists, however, pathway metabolites have long been interested in these novel phytochemicals and have 24.10 Phenylpropanoid and investigated their chemical properties extensively since the 1850s. phenylpropanoid-acetate Studies of natural products stimulated development of the separa- biosynthesis tion techniques, spectroscopic approaches to structure elucidation, and synthetic methodologies that now constitute the foundation of 24.11 Biosynthesis of lignans, lignins, contemporary organic chemistry. -

Characterization of Human TRPA1 and TRPV1 Channels in Response to Naturally Occurring Defensive Compounds
Characterization of human TRPA1 and TRPV1 channels in response to naturally occurring defensive compounds The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Ibarra, Yessenia Michelle. 2013. Characterization of human TRPA1 and TRPV1 channels in response to naturally occurring defensive compounds. Doctoral dissertation, Harvard University. Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:11156673 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Other Posted Material, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#LAA © 2013 – Yessenia Michelle Ibarra All rights reserved. Dissertation Advisor: David E. Clapham Yessenia Michelle Ibarra Characterization of human TRPA1 and TRPV1 channels in response to naturally occurring defensive compounds Abstract The transient receptor potential channels, ankyrin 1 (TRPA1) and vanilloid 1 (TRPV1), are non-selective cation-permeable channels that have retained their function as chemical sensors since their first appearance in metazoan species several hundred million years ago. In vertebrates, TRP channels have evolved multiple functions which make it difficult to understand exactly how they transmit signals to the brain that are interpreted very differently. For example, TRPA1 and TRPV1 are sensitive to various chemicals and activation of these channels produce sensations with opposing effects. Pain is felt when TRPV1 is activated by spider toxins, but activation by plant cannabidiol results in a pain-relieving sensation. Similarly, TRPA1 activation by delta- tetrahydrocannabinol is reported to relieve symptoms of pain, but TRPA1 activation by the active ingredient in wasabi results in a repulsive or noxious sensation. -

Download Download
Journal of International Society for Food Bioactives Nutraceuticals and Functional Foods Review J. Food Bioact. 2020;10:32–46 Chemistry and biochemistry of dietary carotenoids: bioaccessibility, bioavailability and bioactivities Cheng Yanga,b, Lianfu Zhanga,b and Rong Tsaoc* aState Key Laboratory of Food Science and Technology, Jiangnan University, 1800 Lihu Avenue, Wuxi, Jiangsu, 214122, China bSchool of Food Science and Technology, Jiangnan University, 1800 Lihu Avenue, Wuxi, Jiangsu, 214122, China cGuelph Research and Development Centre, Agriculture and Agri-Food Canada, 93 Stone Road West, Guelph, Ontario N1G 5C9, Canada *Corresponding author: Rong Tsao, Guelph Research and Development Centre, Agriculture and Agri-Food Canada, 93 Stone Road West, Guelph, Ontario N1G 5C9, Canada. Tel: +1 226 217 8180. Fax: +1 226 217 8183. E-mail: [email protected] DOI: 10.31665/JFB.2020.10225 Received: April 10, 2020; Revised received & accepted: June 26, 2020 Citation: Yang, C., Zhang, L., and Tsao, R. (2020). Chemistry and biochemistry of dietary carotenoids: bioaccessibility, bioavailability and bioactivities. J. Food Bioact. 10: 32–46. Abstract Carotenoids are one of the major food bioactives that provide humans with essential vitamins and other critically important nutrients that contribute to the maintenance of human health. This review provides a summary of the most current literature data and information on most recent advances in dietary carotenoids and human health research. Specifically, it addresses the occurrence and distribution of carotenoids in different dietary sources, the effect of food processing and impact of gastrointestinal digestion on the bioaccessibility, bioavailability and bioac- tivities of dietary carotenoids. Emphasis is placed on the antioxidant and anti-inflammatory effects of carotenoids and their optical/geometric isomers and ester forms, and the molecular mechanisms behind these actions. -

Carotene, Lutein, and Zeaxanthin in Eye Health and Disease
antioxidants Review A Mechanistic Review of β-Carotene, Lutein, and Zeaxanthin in Eye Health and Disease Fatima Tuj Johra, Asim Kumar Bepari , Anika Tabassum Bristy and Hasan Mahmud Reza * Department of Pharmaceutical Sciences, School of Health and Life Sciences, North South University, Bashundhara R/A, Dhaka 1229, Bangladesh; [email protected] (F.T.J.); [email protected] (A.K.B.); [email protected] (A.T.B.) * Correspondence: [email protected]; Tel.: +880-255668200 (ext. 1954) Received: 12 September 2020; Accepted: 22 October 2020; Published: 26 October 2020 Abstract: Carotenoids are natural lipid-soluble antioxidants abundantly found as colorful pigments in fruits and vegetables. At least 600 carotenoids occur naturally, although about 20 of them, including β-carotene, α-carotene, lycopene, lutein, zeaxanthin, meso-zeaxanthin, and cryptoxanthin, are detectable in the human blood. They have distinct physiological and pathophysiological functions ranging from fetal development to adult homeostasis. β-carotene is a precursor of vitamin A that essentially functions in many biological processes including vision. The human macula lutea and eye lens are rich in lutein, zeaxanthin, and meso-zeaxanthin, collectively known as macular xanthophylls, which help maintain eye health and prevent ophthalmic diseases. Ocular carotenoids absorb light from the visible region (400–500 nm wavelength), enabling them to protect the retina and lens from potential photochemical damage induced by light exposure. These natural antioxidants also aid in quenching free radicals produced by complex physiological reactions and, consequently, protect the eye from oxidative stress, apoptosis, mitochondrial dysfunction, and inflammation. This review discusses the protective mechanisms of macular xanthophylls in preventing eye diseases such as cataract, age-related macular degeneration, and diabetic retinopathy. -

Research Article Simultaneous Determination of Catalpol, Aucubin
Hindawi Publishing Corporation Journal of Analytical Methods in Chemistry Volume 2016, Article ID 4956589, 6 pages http://dx.doi.org/10.1155/2016/4956589 Research Article Simultaneous Determination of Catalpol, Aucubin, and Geniposidic Acid in Different Developmental Stages of Rehmannia glutinosa Leaves by High Performance Liquid Chromatography Yanjie Wang,1,2 Dengqun Liao,1 Minjian Qin,2 and Xian’en Li1 1 Institute of Medicinal Plant Development, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100193, China 2Department of Resources Science of Traditional Chinese Medicines, China Pharmaceutical University, Nanjing 210009, China Correspondence should be addressed to Xian’en Li; [email protected] Received 29 March 2016; Revised 15 May 2016; Accepted 8 June 2016 Academic Editor: Giuseppe Ruberto Copyright © 2016 Yanjie Wang et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Although R. glutinosa roots are currently the only organ source in clinics, its leaves are a potential supplement for the roots especially in extraction of some important bioactive compounds. Our early work found that the contents of catalpol and total iridoid glycosides varied among different developmental stages of R. glutinosa leaves. Aucubin and geniposidic acid, the abundant major bioactive compounds in Eucommia ulmoides and Gardenia jasminoides, respectively, were found present in R. glutinosa roots, however, and have not been analyzed in its leaves. In this paper, we aimed to determine contents of these three iridoid glycosides in different developmental stages of R. glutinosa leaves using the optimized HPLC-UV conditions. -

Epoxidation of Terpenes with Molecular Catalysts in Homogeneous Phase
Technische Universität München Lehrstuhl für Molekulare Katalyse Epoxidation of Terpenes with Molecular Catalysts in Homogeneous Phase Typhène Michel Vollständiger Abdruck der von der Fakultät für Chemie der Technischen Universität München zur Erlangung des akademischen Grades eines Doktors der Naturwissenschaften genehmigten Dissertation. Vorsitzende: Univ. – Prof. Dr. K. Köhler Prüfer der Dissertation: 1. Univ. – Prof. Dr. F. E. Kühn 2. Univ. – Prof. Dr. V. Sieber Die Dissertation wurde am 22.10.2012 bei der Technischen Universität München eigereicht und durch die Fakultät für Chemie am 26.11.2012 angenommen. A mes parents, mon frère, ma sœur et mes amis Toute action naturelle est engendrée par la nature, de la plus courte façon que l’on puisse trouver. Human subtlety… will never devise an invention more beautiful, more simple or more direct than does nature, because in her inventions nothing is lacking, and nothing is superfluous. Leonardo da Vinci Die vorliegende Arbeit entstand in der Zeit von Oktober 2009 bis Oktober 2012 am Anorganisch-chemischen Institute der Technischen Universität München I would like to express my deep gratitude to my academic supervisor Prof. Dr. Fritz E. Kühn For his continuous supervision, encouragement and trust in my work. Acknowledgements I will first address my most sincere thanks to Prof. F. E. Kühn for allowing me to perform my PhD in his groups, for his trust in my research and his advices and to Dr. Mirza Cokoja, for his scientific advices and continuous help during my PhD on correcting my manuscripts. Fraunhofer Institut and Prof. V. Sieber are acknowledged for their financial support. I heartily thank my family: my parents, my sister (Alison) and my brother (Clément) for their continuous love and encouragement throughout my studies and my personal life. -
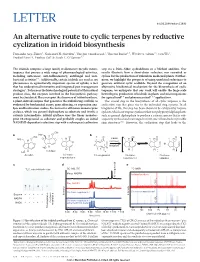
An Alternative Route to Cyclic Terpenes by Reductive Cyclization in Iridoid Biosynthesis
LETTER doi:10.1038/nature11692 An alternative route to cyclic terpenes by reductive cyclization in iridoid biosynthesis Fernando Geu-Flores1, Nathaniel H. Sherden1, Vincent Courdavault2, Vincent Burlat3,4, Weslee S. Glenn1,5, Cen Wu6, Ezekiel Nims5{, Yuehua Cui6 & Sarah E. O’Connor1,7 The iridoids comprise a large family of distinctive bicyclic mono- step via a Diels–Alder cycloaddition or a Michael addition. Our terpenes that possess a wide range of pharmacological activities, results illustrate how a short-chain reductase was recruited as including anticancer, anti-inflammatory, antifungal and anti- cyclase for the production of iridoids in medicinal plants. Further- bacterial activities1–4. Additionally, certain iridoids are used as sex more, we highlight the prospects of using unrelated reductases to pheromones in agriculturally important species of aphids, a fact generate artificial cyclic scaffolds. Beyond the recognition of an that has underpinned innovative and integrated pest management alternative biochemical mechanism for the biosynthesis of cyclic strategies5. To harness the biotechnological potential of this natural terpenes, we anticipate that our work will enable the large-scale product class, the enzymes involved in the biosynthetic pathway heterologous production of iridoids in plants and microorganisms must be elucidated. Here we report the discovery of iridoid synthase, for agricultural5–8 and pharmaceutical1–4,9 applications. a plant-derived enzyme that generates the iridoid ring scaffold, as The crucial step in the