Umpolung Strategy: Advances in Catalytic C-C Bond Formations
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Sep. 5, Hydroacylation by Brandon Reinus
Hydroacylation and Related Topics Dong Group Seminar Brandon Reinus Wed, Sept. 5th 2012 Why? ¡ Looking at the reaction, it is a highly atom- economical approach to synthesizing ketones ¡ Umpolung (ex: deprotonating dithioacetals) ¡ Using acrylate derivatives generates a 1,4 diketone relationship, a hard relationship to establish using classical organic synthesis. Presentation Overview 1. Hydroformylation (extremely brief) 2. Rh-Catalyzed Hydroacylation ¡ Intramolecular ¡ Intermolecular ¡ Other 3. NHC Catalyzed Hydroacylation ¡ Benzoin reaction ¡ Stetter reaction ¡ other Part 1 : Background Reppe Roelen Science of Synthesis, Stereoselective Synthesis 1, 2011, pg.409 Hydroformylation Metal-Organic Cooperative Catalysis Park et al. Introduction SCHEME 2 Among the many elegant examples of transition metal cat- alyzed activation of C-HandC-Cbonds,1 chelation-as- sisted protocols have recently attracted increasing attention in organometallic chemistry. Directed metalation processes have been demonstrated by valuable applications in organic synthesis, showing remarkable efficiency and chemoselectivity.2 In general, a chelation-assisted proto- col facilitates the formation of either the kinetically or ther- modynamically favored five- or six-membered metallacycle; aprepositionedcoordinatinggroupinducesspatialproxim- ing decarbonylation, their structures are too specific to apply ity between the C-HorC-Cbondsandthetransitionmetal for common aldehydes. center.1,2 Despite the magnificent usefulness in activating otherwise stable C-HandC-Cbonds,amajordrawbackof -

UMPOLUNG in REACTIONS CATALYZED by THIAMINE PYROPHOSPHATE DEPENDENT ENZYMES Umpolung En Reacciones Catalizadas Por Enzimas Dependientes De Pirofosfato De Tiamina
Ciencia, Ambiente y Clima, Vol. 2, No. 2, julio-diciembre, 2019 • ISSN (impreso): 2636-2317 • ISSN (en línea): 2636-2333 DOI: https://doi.org/10.22206/cac.2019.v2i2.pp27-42 UMPOLUNG IN REACTIONS CATALYZED BY THIAMINE PYROPHOSPHATE DEPENDENT ENZYMES Umpolung en reacciones catalizadas por enzimas dependientes de pirofosfato de tiamina Carlos José Boluda Emily Soto Instituto Tecnológico de Santo Domingo (INTEC), Instituto Tecnológico de Santo Domingo (INTEC), Área de Ciencias Básicas y Ambientales, Av. de Los Área de Ciencias Básicas y Ambientales Próceres 49, Santo Domingo, República Dominicana Correo-e: [email protected] *Corresponding author: Carlos J. Boluda Darah de la Cruz Correo-e: [email protected] Instituto Tecnológico de Santo Domingo (INTEC), Carolina Juncá Área de Ciencias Básicas y Ambientales Instituto Tecnológico de Santo Domingo (INTEC), Correo-e: [email protected] Área de Ciencias Básicas y Ambientales Anny Peña Correo-e: [email protected] Instituto Tecnológico de Santo Domingo (INTEC), Área de Ciencias Básicas y Ambientales Correo-e: [email protected] Recibido: 25/9/2019 • Aprobado: 19/10/2019 Cómo citar: Boluda, C. J., Juncá, C., Soto, E., de la Cruz, D., & Peña, A. (2019). Umpolung in reactions catalyzed by thiamine pyrophos- phate dependent enzymes. Ciencia, Ambiente Y Clima, 2(2), 27-42. Doi: https://doi.org/10.22206/cac.2019.v2i2.pp27-42 Abstract Resumen The temporal exchange of the electrophilic/nucleophilic El intercambio temporal del carácter electrofílico/nucleofí- character of an atom by chemical manipulation is known lico de un átomo mediante manipulación química, es cono- in organic chemistry as umpolung. This inversion of polarity cido con el vocablo alemán de umpolung. -

Brittain-DR-1965-Phd-Thesis.Pdf
POLYMETHYLENE PYRIDINES , A thesis submitted by David Robert Brittain in partial fulfilment of the requirements for the degree of DOCTOR OP PHILOSOPHY in the University of London Organic Chemistry Department, dune, 1965. Imperial College, LONDON, S.W.7. ABSTRACT This thesis describes a series of attempts to syn- thesise 2,5- and 1,4-polymethylene bridged pyridines. Nuclear magnetic resonance theory predicts that protons, which are held directly over an aromatic ring, will be abnormally shielded compared with protons in aliphatic straight-chain hydrocarbons. This prediction has been verified for the central methylene protons of paracyclo- phanes. The degree of shielding, expressed in terms of the distance from the aromatic ring, is a measure of the induced ring current and hence the aromaticity of the benzene ring. Similar measurements upon 2,5- or 1,4— polymethylene bridged pyridines would make it possible to determine the degree of aromaticity of the pyridine ring relative to benzene. A review of the subject of aromaticity is presented in which special reference has been made to its inter- pretation by nuclear magnetic resonance. The synthetic work has not been brougL.t to a truly satisfactory conclusion. However, the synthetic routes to 2,5-dialkylpyridines have been thoroughly investigated and a wide variety of such compounds prepared. The functional groups at the ends of the alkyl chains have been varied in an effort to produce a derivative which would cyclise to give a 2,5-bridged pyridine. The attempted intramolecular oxidative coupling of 2,5-dihex- 51 -ynylpyridine received much attention. In the attempts to obtain a 1,4-bridged pyridine, two tricyclic compounds, each containing two quaternised pyridine rings linked by polymethylene chains, were obtained. -
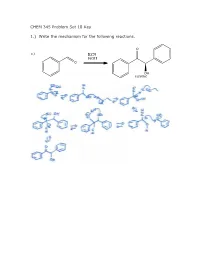
CHEM 345 Problem Set 18 Key 1.) Write the Mechanism for The
CHEM 345 Problem Set 18 Key 1.) Write the mechanism for the following reactions. O a.) KCN EtOH O OH racemic 1.) Write the mechanism for the following reactions. b.) KCN AcOH O NC OH racemic O c.) N S R O NEt3 OH racemic 2.) What is the structure of AcOH?Why does changing the solvent from EtOH to AcOH make such a big difference? O OH AcOH acetic acid The pKa of acetic acid is approximately 5. The pKa of ethanol is approximately 15. When you take a proton off of ethanol, you generate ethoxide which is about 1010 times stronger of a base than acetate. 3.) Give two instances when you need to use the thiazolium salt and triethylamine rather than KCN and EtOH. If the aldehydes contain an enolizable proton then you cannot use KCN/EtOH, instead you must use the thiazolium. Also, if the electrophile is a Michael acceptor to give a 1,4 dicarbonyl, then the thiazolium catalyst should be used. 4.) Break the following compound down as far as you can using Aldol, Michael, and Claisen reactions. Above each retrosynthetic arrow, write the name of the reaction. O HO O HO Aldol O Michael HO O O HO O Aldol O O Aldol HO O O HO Michael O O O Aldol There are other possibilities for order. O O HO Aldol O O 5.) Synthesize the following molecules. All carbons in the molecules must come from benzene or compounds with 5C’s or less. a.) O H2SO4 O HNO3 O2N AlCl3 O O SOCl2 HO Cl H2CrO4 1.) BuLi, Et2O + O 2.) H3O HO b.) O O O Cl + H3O NaOEt, EtOH O O O O Cl O AlCl3 Cl Cl AlCl3 Cl2 c.) O OMe NaOMe MeOH O O 1.) POCl3, DMF 2.) H2O OMe OMe O AlCl3 MeI Cl ONa HCl NaOH ZnHg 1.) NaOH + mcpba O 2.) H3O O OH O O AlCl3 Cl 6.) Write the mechanism for the following reactions. -

Benzoin and Stobbe Reactions
____________________________________________________________________________________________________ Subject Chemistry Paper No and Title 9; Organic Chemistry-III (Reaction Mechanism-2) Module No and Title 21; Named Reactions: Benzoin Condensation and Stobbe Condensation Module Tag CHE_P9_M21 CHEMISTRY Paper 9: Organic Chemistry-III (Reaction Mechanism-2) Module NO. 21: Benzoin Condensation and Stobbe Condensation ____________________________________________________________________________________________________ TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction 3. Benzoin Condensation 3.1 Mechanism of Benzoin Condensation 3.2 Characteristics of Benzoin Condensation 3.3 Reactions of Benzoin 4. Stobbe Condensation 4.1 Mechanism of Stobbe Condensation 4.2 Characteristics of Stobbe Condensation 4.3 Few Examples of Stobbe Condensation 5. Summary CHEMISTRY Paper 9: Organic Chemistry-III (Reaction Mechanism-2) Module NO. 21: Benzoin Condensation and Stobbe Condensation ____________________________________________________________________________________________________ 1. Learning Outcomes After studying this module, you shall be able to: Know what are Benzoin condensation and Stobbe condensation reactions Learn mechanism of Benzoin and Stobbe condensation reactions Know about the role of CN- ion in Benzoin condensation Identify the products of reduction and oxidation of Benzoin condensation Understand the product formation in Benzoin and Stobbe condensation. 2. Introduction A condensation reaction, also commonly referred to as dehydration -

Nuclear Magnetic Resonance Approaches in the Study of 2-Oxo Acid Dehydrogenase Multienzyme Complexes— a Literature Review
Molecules 2013, 18, 11873-11903; doi:10.3390/molecules181011873 OPEN ACCESS molecules ISSN 1420-3049 www.mdpi.com/journal/molecules Review Nuclear Magnetic Resonance Approaches in the Study of 2-Oxo Acid Dehydrogenase Multienzyme Complexes— A Literature Review Sowmini Kumaran 1, Mulchand S. Patel 2 and Frank Jordan 1,* 1 Department of Chemistry, Rutgers University, Newark, NJ 07102, USA 2 Department of Biochemistry, School of Medicine and Biomedical Sciences, State University of New York at Buffalo, Buffalo, NY 14214, USA * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +1-973-353-5470; Fax: +1-973-353-1264. Received: 30 July 2013; in revised form: 14 September 2013 / Accepted: 16 September 2013 / Published: 26 September 2013 Abstract: The 2-oxoacid dehydrogenase complexes (ODHc) consist of multiple copies of three enzyme components: E1, a 2-oxoacid decarboxylase; E2, dihydrolipoyl acyl-transferase; and E3, dihydrolipoyl dehydrogenase, that together catalyze the oxidative decarboxylation of 2-oxoacids, in the presence of thiamin diphosphate (ThDP), coenzyme A 2+ + (CoA), Mg and NAD , to generate CO2, NADH and the corresponding acyl-CoA. The structural scaffold of the complex is provided by E2, with E1 and E3 bound around the periphery. The three principal members of the family are pyruvate dehydrogenase (PDHc), 2-oxoglutarate dehydrogenase (OGDHc) and branched-chain 2-oxo acid dehydrogenase (BCKDHc). In this review, we report application of NMR-based approaches to both mechanistic and structural issues concerning these complexes. These studies revealed the nature and reactivity of transient intermediates on the enzymatic pathway and provided site-specific information on the architecture and binding specificity of the domain interfaces using solubilized truncated domain constructs of the multi-domain E2 component in its interactions with the E1 and E3 components. -

Benzoin Condensation
GENERAL ARTICLE Benzoin Condensation The Cyanide Connection with Tapioca and Vitamin B1 Gopalpur Nagendrappa Benzoin condensation is an important carbon–carbon bond forming reaction. It is achieved by generating an acyl anion equivalent from one aldehyde molecule which adds to a second aldehyde molecule. The reaction is traditionally catalysed by a cyanide ion. Cyanohydrin anion is the first intermediateandistheprecursortotheacylanionequivalent. G Nagendrappa, retired Cyanohydrins are found in plants as glycosides. A reaction from Bangalore Univer- completely analogous to benzoin condensation occurs in our sity, Bangalore, is body, which however neither involves cyanohydrin interme- presently Professor and diate nor is catalysed by cyanide ion. It is catalysed by the Head of the Department of Medicinal Chemistry, Sri thiazolium moiety of the co-enzyme thiamine pyrophosphate Ramachandra University, (TPP). This article shows the common links and inclusive Porur, Chennai. His main chemistry aspects among cyanohydrin formation, naturally work is in the areas of occurring cyanohydrins, conversion of cyanohydrins to ben- organosilicon chemistry, organic synthesis, reaction zoins/acyloins, the role of vitamin B1 (thiamine) andthe use of mechanism and synthetic thiazolium compounds in benzoin/acyloin condensation. methodologies. Introduction Cyano Group in Natural Products 1. Cyanoglycosides Hydrogen cyanide is a deadly poisonous substance. A variety of plants produce it, though in the hidden form of cyanoglycosides, the sugar derivatives of cyanohydrins. Cyanohydrins are formally the products of HCN addition to ketones or aldehydes, the addi- Keywords tion being reversible. Cyanoglycosides hydrolyse enzymatically Benzoin condensation, acyloin as well as nonenzymatically in the body to sugar and cyanohy- condensation, cyanoglycosides, cyanohydrins, vitamin B cataly- drins, which release hydrogen cyanide, Scheme 1. -

JUN 1 51998 Science
Efforts Toward the Syntheses of Natural Products: Part A: Paeoniflorin Part B: (+)-Taxusin A thesis presented by Rebecca J. Carazza B.S. Chemistry University of Massachusetts, Amherst, 1993 Submitted to the Department of Chemistry in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Chemistry at the Massachusetts Institute of Technology June 1998 © 1998 Massachusetts Institute of Technology. All rights reserved. SinatureofAuthor: SignatureT- -~- -- .. -uof -Author: -- I- ----- D4pdrtment o('ihemistry May 26, 1998 Certified by: ,Scott C. Virgil Thesis Advisor Acceuted bv: Dietmar Seyferth Chairman, Departmental Committee on Graduate Students O-V .Z\ . JUN 1 51998 Science UR PAES This doctoral thesis has been examined by a committee of the Department of Chemistry as follows: Professor Rick L. Danheiser - Chairman Professor Scott C. Virgil / Th/sis Supervisor Professor Peter H. Seeberger Efforts Toward the Syntheses of Natural Products: Part A: Paeoniflorin Part B: (+)-Taxusin by Rebecca J. Carazza Submitted to the Department of Chemistry on May 26, 1998 in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy in Chemistry Massachusetts Institute of Technology ABSTRACT Part A Efforts toward the synthesis of the monoterpene glycoside paeoniflorin (1) are discussed. Optimization of the previous synthetic route was successful. Synthesis of the key cc-diazo intermediate 30 was achieved and the construction of the carbocyclic frame was completed. Key reactions of the strategy involve a ring contraction via a Wolff rearrangement, and formation of the lactone 78 which undergoes diisobutylaluminum hydride reduction followed by acid catalyzed cyclization to the paeoniflorin ring system 80. A new synthetic strategy was initiated to prepare the key [3.2.1]bicyclooctanone 81 using a palladium mediated olefin cyclization of the acyloin substrate 82. -

CHEM 33 Unless You Have Completed the Laboratory and Turned in a Notebook, So Be Sure to Turn in Your Notebook on Time to Your Laboratory Instructor
SANTA CLARA UNIVERSITY SUMMER TERM 2019 ORGANIC CHEMISTRY III CHEMISTRY 33L LABORATORY SYLLABUS INSTRUCTORS Raymond Gipson, Ph.D.; Beatrice Ruhland, Ph.D. TIMES • Monday/Wednesday: 8:00-12:30 p.m. • Monday/Wednesday: 4:30-9:00 p.m. • Tuesday/Thursday: 8:00-12:30 p.m. • Tuesday/Thursday: 4:30-9:00 p.m. Laboratories begin on Monday, July 29th and are held in DS 126 MATERIALS 1. Course syllabus (available on Canvas, Chem33 lab course page or on google sites https://sites.google.com/a/scu.edu/organic-chemistry-laboratory/, and from Copy Craft Printing, 341 Lafayette St.) 2. Laboratory Techniques in Organic Chemistry by Mohrig, Alberg, Hofmeister, Schatz and Hammond, 4th Edition 3. Bound laboratory notebook embossed with "Santa Clara University", Scientific Notebook Company (available from the bookstore); you may use the same notebook as you purchasEd for Chem32L 4. Safety splash goggles 5. Laboratory coat (available from the bookstore) LABORATORY GUIDELINES 1. The laboratory in Daly Science 100 were modernized and redesigned in 1994, with safety as the primary concern. The main improvement in these laboratories was increasing the number of hoods so that each student has a workstation in a hood, two students per hood. This significant enhancement in safety protects everyone because many organic compounds are volatile and using a hood minimizes your exposure to fumes. Therefore, virtually 100% of your chemical work should be performed in a fume hood. 2. To do organic chemistry safely, you should treat all chemicals with respect; glovEs should be worn unless otherwise directed by the instructor. 3. -

Synthesis of a Poison Frog Bioactive Alkaloids Sara Mazeh
Synthesis of a poison frog bioactive alkaloids Sara Mazeh To cite this version: Sara Mazeh. Synthesis of a poison frog bioactive alkaloids. Organic chemistry. Université Grenoble Alpes, 2019. English. NNT : 2019GREAV043. tel-02613846 HAL Id: tel-02613846 https://tel.archives-ouvertes.fr/tel-02613846 Submitted on 20 May 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. THÈSE Pour obtenir le grade de DOCTEUR DE LA COMMUNAUTÉ UNIVERSITÉ GRENOBLE ALPES Spécialité : Chimie organique Arrêté ministériel : 25 mai 2016 Présentée par SARA MAZEH Thèse dirigée par Philippe DELAIR préparée au sein du Laboratoire Département de Pharmaco chimie Moléculaire dans l'École Doctorale Chimie et Sciences du Vivant Synthèse d'alcaloïdes bioactifs issus de batracien Synthesis of a poison frog bioactive alkaloids Thèse soutenue publiquement le 9 décembre 2019, devant le jury composé de : Madame MERCEDES AMAT PROFESSEUR, UNIVERSITE DE BARCELONE - ESPAGNE, Rapporteur Monsieur DAVID AITKEN PROFESSEUR DES UNIVERSITES, UNIVERSITE PARIS-SUD, Rapporteur Monsieur PETER GOEKJIAN PROFESSEUR DES UNIVERSITES, UNIVERSITE LYON 1, Examinateur Madame SANDRINE PY DIRECTRICE DE RECHERCHE, CNRS DELEGATION ALPES, Examinateur Monsieur OLIVIER RENAUDET PROFESSEUR DES UNIVERSITES, UNIVERSITE GRENOBLE ALPES, Président Monsieur PHILIPPE DELAIR MAITRE DE CONFERENCES HDR, UNIVERSITE GRENOBLE ALPES, Directeur de thèse Dedicated to my parents, this simply wouldn’t exist without you. -

Syntheses of Morphine and Codeine (1992 – 2002): Templates for Exploration of Synthetic Tools
Current Organic Synthesis, 2006, 3, 99-120 99 Syntheses of Morphine and Codeine (1992 – 2002): Templates for Exploration of Synthetic Tools L. M. Mascavage#, M. L. Wilson and D. R. Dalton* Department of Chemistry (016-00), Beury Hall, 13th and Norris Streets, Temple University, Philadelphia, PA 19122, USA and Department of Chemistry, Arcadia University, Glenside, PA, 19038, USA Abstract: Morphine (1) and its O-methylated analogue codeine (2), analgesic alkaloids of the opium poppy (Papaver Somniferium), have been targets of organic chemists engaged in synthetic activities for at least half a century. The “first” (Gates) and “most efficient” (Rice) syntheses of morphine (1) and codeine (2) are well known and have been reviewed and analyzed extensively numerous times. However, syntheses of the same two alkaloids that have been reported since 1992 and which have been used as devices to advance the art of organic synthesis are not as widely recognized and they have not been as thoroughly reviewed. Here they are analyzed in the spirit of the use of these two compounds as templates. Further, since both racemic and enantiospecific syntheses are important and since all eight (8) approaches (since 1992) are sufficiently different so as to warrant more tha n superficial examination, they are all considered. H HO 7 H 8 15 6 H H 6 14 N 5 CH3 H 13 NCH3 HO 14 13 9 16 9 O 16 12 15 10 O 10 4 4 11 1, R = H 2, R = CH 1 1 3 RO 3 RO 3 2 2 It is nearly two hundred years since the initial report the ubiquitous standard opium poppy or with cultivars that (1806) of the isolation of morphine (1, R = H) from the might be subsequently generated through genetic unripe seed pods of the opium poppy, Papever somniferum, manipulations so as to maximize production of these or by Friedrich Wihelm Adam Setürner [1], seventy five years related bases. -

Durham E-Theses
Durham E-Theses The synthesis and potential applications of asymmetric silacycles Matthews, Jennifer Louise How to cite: Matthews, Jennifer Louise (1994) The synthesis and potential applications of asymmetric silacycles, Durham theses, Durham University. Available at Durham E-Theses Online: http://etheses.dur.ac.uk/5504/ Use policy The full-text may be used and/or reproduced, and given to third parties in any format or medium, without prior permission or charge, for personal research or study, educational, or not-for-prot purposes provided that: • a full bibliographic reference is made to the original source • a link is made to the metadata record in Durham E-Theses • the full-text is not changed in any way The full-text must not be sold in any format or medium without the formal permission of the copyright holders. Please consult the full Durham E-Theses policy for further details. Academic Support Oce, Durham University, University Oce, Old Elvet, Durham DH1 3HP e-mail: [email protected] Tel: +44 0191 334 6107 http://etheses.dur.ac.uk The Synthesis and Potential Applications of Asymmetric Silacycles The copyright of this thesis rests with the author. No quotation from it should be published without his prior written consent and information derived from it should be acknowledged. Jennifer Louise Matthews, B.Sc. (Hons) Ph.D. Thesis University of Durham November 1994 COPYRIGHT The copyright of this work rests with the author. No quotation from it should be published without prior consent. Information derived from this thesis should be acknowledged. DECLARATION The work contained in this thesis was carried out in the Department of Chemistry at the University of Durham between October 1991 and September 1994.