Arowhenua Contaminants Report-Data-Final
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CEN33 CSI Fish & Game Opihi River Flyer
ACCESS ETIQUETTE • No dogs • No guns Opihi River • No camping • Leave gates as you find them • Stay within the river margins • Do not litter • Respect private property • Avoid disturbing stock or damaging crops • Do not park vehicles in gateways • Be courteous to local landowners and others Remember the reputation of ALL anglers is reflected by your actions FISHING ETIQUETTE • Respect other anglers already on the water • Enquire politely about their fishing plans • Start your angling in the opposite direction • Refer to your current Sports Fishing Guide for fishing regulations and bag limits A successful angler on the Opihi River Pamphlet published in 2005 Central South Island Region Cover Photo: Lower Opihi River upstream of 32 Richard Pearse Drive, PO Box 150, Temuka, New Zealand State Highway 1 Bridge Telephone (03) 615 8400, Facsimile (03) 615 8401 Photography: by G. McClintock Corporate Print, Timaru Central South Island Region THE OPIHI RIVER Chinook salmon migrate into the Opihi River ANGLING INFORMATION usually in February and at this time the fishing pressure in the lower river increases significantly. FISHERY The Opihi River supports good populations of As a result of warm nor-west rain and snow melt both chinook salmon and brown trout. In the The Opihi River rises in a small modified wetland waters from the mouth to about the State of approximately 2 hectares at Burkes Pass and the larger Rakaia and Rangitata Rivers often flood and during these times the spring fed Opihi Highway 1 bridge there is a remnant population flows in an easterly direction for about 80 km to of rainbow trout, survivors of Acclimatisation enter the Pacific Ocean 10 km east of Temuka. -

New Zealand Gazette
~umb.· 127. 3721 THE NEW ZEALAND GAZETTE WELLINGTON, THURSDAY, DECEMBER 19, 1940. Additional Land at Belfa,;;t taken far the Piirposes of the Additional Land taken far Post and Telegraph Purposes in the Hiirunui-Waitaki Railway. City of Christchurch. [ L.S.] GALWAY, Governor-General. [L.S.] GALWAY, Governor-General. A PROCLAMATION. A PROCLAl'VIATION. HEREAS it has been found desirable for the use, con N pursuance and exercise of the powers and authorities W venience, and enjoyment of the Hurunui-Waitaki I vested in me by the Public Works Act, 1928, and of Ra.ilway to take further land at Belfast in addition to land every other power and authority in anywise enabling me in previously acquired for the purposes of the said railway : this behalf, I, George Vere Arundell, Viscount Galway, Now, therefore, I, George Vere Arundel!, Viscount Galway, Governor-General of the Dominion of New Zealand, do Governor-General of the Dominion of New Zealand, in hereby proclaim and declare that the land described in the exercise of the powers and authorities conferred on me by Schedule hereto is hereby taken for post and telegraph sections thirty-four and two hundred and sixteen of the purposes; and I do also declare that this Proclamation shall Public Works Act, 1928, and of every other power and take effect on and after the twenty-third day of December, authority in anywise enabling me in this behalf, do hereby one thousand nine hundred and forty. proclaim and declare that the land described in the Schedule hereto is hereby taken for the purposes above mentioned. -

Ecosystem Services Review of Water Storage Projects in Canterbury: the Opihi River Case
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Lincoln University Research Archive Ecosystem Services Review of Water Storage Projects in Canterbury: The Opihi River Case By Dr Edward J. S. Hearnshaw1, Prof Ross Cullen1 and Prof Ken F. D. Hughey2 1Faculty of Commerce and 2Faculty of Environment, Society and Design Lincoln University, New Zealand 2 Contents Executive Summary 5 1.0 Introduction 6 2.0 Ecosystem Services 9 3.0 The Opihi River and the Opuha Dam 12 4.0 Ecosystem Services Hypotheses 17 4.1 Hypotheses of Provisioning Ecosystem Services 17 4.2 Hypotheses of Regulating Ecosystem Services 19 4.3 Hypotheses of Cultural Ecosystem Services 20 5.0 Ecosystem Services Indicators 25 5.1 Indicators of Provisioning Ecosystem Services 27 5.2 Indicators of Regulating Ecosystem Services 36 5.3 Indicators of Cultural Ecosystem Services 44 6.0 Discussion 49 6.1 Ecosystem Services Index Construction 51 6.2 Future Water Storage Projects 56 7.0 Acknowledgements 58 8.0 References 59 3 4 Ecosystem Services Review of Water Storage Projects in Canterbury: The Opihi River Case By Dr Edward J. S. Hearnshaw1, Prof Ross Cullen1 and Prof Ken F. D. Hughey2 1Commerce Faculty and 2Environment, Society and Design Faculty, Lincoln University, New Zealand When the well runs dry we know the true value of water Benjamin Franklin Executive Summary There is an ever‐increasing demand for freshwater that is being used for the purposes of irrigation and land use intensification in Canterbury. But the impact of this demand has lead to unacceptable minimum river flows. -

Off-Road Walking and Biking Strategy 2012 to 2032
Off-Road Walking and Biking Strategy 2012 to 2032 Prepared by Bill Steans, Parks and Recreation Manager and Gary Foster, Parks Liaison Officer February 2012 Contents Executive Summary .................................................................................................... 4 1 Context ............................................................................................................. 7 1.1 Purpose of the Off- Road Biking and Walking Strategy ............................................. 8 1.2 How Walkways and Cycleways Contribute to the Delivery of Community Outcomes .... 8 1.3 A Vision for Off-Road Walkways and Cycleways ...................................................... 8 1.4 Benefits of Walkways and Cycleways ..................................................................... 9 1.5 Statutory Requirements for Walkways and Cycleways .............................................. 9 1.6 Other Document Linkages .................................................................................. 10 1.7 Walkways and Cycleways Covered by Strategy ..................................................... 10 1.8 Future Provision and Development ...................................................................... 10 1.9 Annual Maintenance Costs ................................................................................. 11 1.10 Maps ................................................................................................................ 11 2 Current Provision ......................................................................................... -
140828 Tas Update TRM.Xlsx
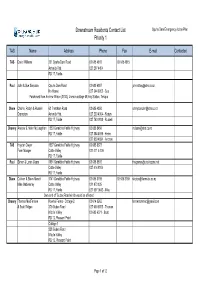
Downstream Residents Contact List Opuha Dam Emergency Action Plan Priority 1 TAS Name Address Phone Fax E-mail Contacted TAS David Williams 231 Opuha Dam Road 03 685 4818 03 685 4815 Ashwick Flat 027 297 4454 RD 17, Fairlie Paul John & Sue Simpson Opuha Dam Road 03 680 6897 [email protected] No House 027 344 8902 - Sue Purchased from Andrew Wilson (2013), Lives in cottage Mt Hay Station, Tekapo Diane Charlie, Robyn & Russell 62 Trentham Road 03 685 4858 [email protected] Crampton Ashwick Flat 027 233 4364 - Robyn RD 17, Fairlie 027 740 9988 - Russell Chonny Andrew & Helen McLaughlan 1283 Geraldine Fairlie Highway 03 685 8456 [email protected] RD 17, Fairlie 027 354 4698 - Helen 027 950 4894 - Andrew TAS Hayden Dwyer 1537 Geraldine Fairlie Highway 03 685 8673 Farm Maager Cattle Valley 027 471 5 736 RD 17, Fairlie Paul Simon & Loren Geary 1891 Geraldine Fairlie Highway 03 685 8815 [email protected] Cattle Valley 027 414 8104 RD 17, Fairlie Diane Colleen & Steve Marett 1741 Geraldine Fairlie Highway 03 685 8789 03 685 8789 [email protected] Mike Mabwinney Cattle Valley 021 873 835 RD 17, Fairlie 027 507 9642 - Mike Own land off Gudex Road which would be affected Chonny Thomas MacFarlane Kowhai Farms - Cottage 2 03 614 8262 [email protected] & Scott Ridgen 379 Gudex Road 027 600 8555 - Thomas Middle Valley 03 685 6071 - Scott RD 12, Pleasant Point Cottage 1 238 Gudex Road Middle Valley RD 12, Pleasant Point Page 1 of 12 Downstream Residents Contact List Opuha Dam Emergency Action Plan Priority 1 TAS Name Address -

Washdyke Lagoon WILDLIFE REFUGE
Phar Lap RaceRacewaway TO CHRISTCHURCH 1 Washdyke Industrial Area Washdyke Lagoon WILDLIFE REFUGE Y LE GOD BEAUMONT E ELLESMER GRANTLEA L Dashing RockRockss OCH ALPI NE E R 1 N AINVIEW Walkway MOUNT EV ERS L EY VILLAGE C GLAM L Gleniti YD STIRLIN E I C S MOOR AR G R LINCOL E D E BALMORAL IN B N U E BRAEMA CLIMI R G H A R R G Y L E SHORT R B CEDA E LL B IR D Highfield Aorangi PaParkrk Ashbury Park Blackett’s O RI Golf Course E Southern TrTrusust Lighthouse L SDALE PARK VIEW T ELM O I H N NU S U Events Centre RAI EY XL UK R L OXBUR W E GH O Y L WAI L O JONAS WI N ON R O M CH TH CA AW A H M TE PB WA A HA E IP U LL O HILLSDE R RT I ST JOHN’S C N W O L OO PrimePort LIN LYSAGHT D ANSCOMBE G BR KARAKA BA Centennial Y HILL Park Westend PAIGNTON THE TERRA H U CE G PaParkrk GUTHRIE H THOMAS Lough PaParkrk C H i SHERRATT A P gy E Alpine Ener L DE T A STUAR L W E L L I N G T SchoolSc O hool Park N W Sacred Heart A TLINT L BasilicaBasilica IN R G U S HectorHectorss T S O E N L O’NEILO’N L L VINNELVINNELL M Coastal E BABBING E I M LL ORIAO R I T A L alklkway E WWa S ERSR A M V SOSO E M A RK Botanic ET Gardens TAY LOR K K E E R PPaatitititi I I I CH T T CAMPBELL H H A R D CBay PPooolol S CemeteCemetery Point Caroline Bay COOPERS 1 Saltwater S I MMO RedruthRedruth PaParrkk H Penguin NS AR Boardwalkalkss Creek Walkway T Viewing Skateboard Park Mini Golf Area BEVERLEY HILL Aviary Disc Golf Soundshell PrimePort BA Y HILLPIAZZA Otipua Wetlands Tuhawaiki JackJacks Point BA TO DUNEDIN YVIEW Supermarket Pharmacy Bus Station Hospital CBay Pool Dog Park BusBu -

Hearing Panel Decision Report
Timaru District Council Timaru Urban Growth Strategy Hearing Panel Decision Report 10 April 2018 2 King George Place - PO Box 522 Timaru 7940 - Telephone 03 687 7200 Page 1 1. BACKGROUND: STRATEGY DEVELOPMENT In October 2014 the Timaru District Council resolved that a growth management strategy be prepared for the district. The purpose of the GMS was to provide an overarching framework for managing growth, and to inform land-use zoning and integrate land-use planning and infrastructure provision, including the staging and allocation of infrastructure expenditure. The intent was to provide a strategic management response to several key challenges facing the district over the next 30 years. Those challenges include: • Modest household growth projections and an increase in older population cohorts; • A history of incremental and unconsolidated rural residential development; and • The need to provide an integrated approach to infrastructure provision and sustainable land supply. The GMS is a non-statutory document but will inform Council documents such as Activity Management Plans, and the strategic priorities of the Long-Term Plan. The GMS will also inform the preparation of a reviewed Timaru District Plan. We note that the District Plan is to implement (‘give effect’ to) the relevant provisions of the Canterbury Regional Policy Statement (2013). We were advised that a series of background reports identified the key issues/constraints to urban growth in the area and informed the development of the GMS. In addition, the GMS had been informed by: • The Canterbury Regional Policy Statement and the principles of relevance in the Mahaanui Iwi Management Plan (2013); and • Council strategic planning and policy, including the Long-term Plan, Infrastructure Strategy, Timaru District Plan and Parks Strategy. -
South Island Fishing Regulations for 2020
Fish & Game 1 2 3 4 5 6 Check www.fishandgame.org.nz for details of regional boundaries Code of Conduct ....................................................................4 National Sports Fishing Regulations ...................................... 5 First Schedule ......................................................................... 7 1. Nelson/Marlborough .......................................................... 11 2. West Coast ........................................................................16 3. North Canterbury ............................................................. 23 4. Central South Island ......................................................... 33 5. Otago ................................................................................44 6. Southland .........................................................................54 The regulations printed in this guide booklet are subject to the Minister of Conservation’s approval. A copy of the published Anglers’ Notice in the New Zealand Gazette is available on www.fishandgame.org.nz Cover Photo: Jaymie Challis 3 Regulations CODE OF CONDUCT Please consider the rights of others and observe the anglers’ code of conduct • Always ask permission from the land occupier before crossing private property unless a Fish & Game access sign is present. • Do not park vehicles so that they obstruct gateways or cause a hazard on the road or access way. • Always use gates, stiles or other recognised access points and avoid damage to fences. • Leave everything as you found it. If a gate is open or closed leave it that way. • A farm is the owner’s livelihood and if they say no dogs, then please respect this. • When driving on riverbeds keep to marked tracks or park on the bank and walk to your fishing spot. • Never push in on a pool occupied by another angler. If you are in any doubt have a chat and work out who goes where. • However, if agreed to share the pool then always enter behind any angler already there. • Move upstream or downstream with every few casts (unless you are alone). -

Orari-Temuka-Opihi-Pareora Water Zone Management Committee
ORARI-TEMUKA-OPIHI-PAREORA WATER ZONE MANAGEMENT COMMITTEE on Monday 5 September 2016 3pm Council Chamber Timaru District Council Timaru ORARI-OPIHI-PAREORA WATER ZONE MANAGEMENT COMMITTEE Notice is hereby given that an Orari-Temuka-Opihi-Pareora Water Zone Management Committee meeting will be held on Monday 5 September 2016 at 3pm in the Council Chamber, Timaru District Council, 2 King George Place, Timaru. Committee Members: John Talbot (Chairman), David Caygill, Kylee Galbraith, John Henry, Mandy Home, Ivon Hurst, Richard Lyon, Hamish McFarlane, James Pearse, Ad Sintenie, Mark Webb and Evan Williams ORARI-TEMUKA-OPIHI-PAREORA WATER ZONE MANAGEMENT COMMITTEE 5 SEPTEMBER 2016 1 Apologies 2 Register of Interest 3 1 Confirmation of Minutes 4 Facilitator Update 5 Community Forum 6 6 Catchment Group Update 7 Washdyke Taskforce Update 8 Groundwater ecosystems: What’s living in our groundwater? 9 8 Immediate Steps Biodiversity Projects 10 18 Immediate Steps Biodiversity Fund Review 11 Level of Protection/Security of Water Supplies in OTOP Zone – TDC Water Services Operations Engineer 12 Regional Committee Update 13 Close ORARI-TEMUKA-OPIHI-PAREORA WATER ZONE MANAGEMENT COMMITTEE FOR THE MEETING OF 5 SEPTEMBER 2016 Report for Agenda Item No 3 Prepared by Joanne Brownie Secretary Confirmation of Minutes – Committee Meeting 1 August 2016 ___________________________ Minutes of the August Committee meeting. Recommendation That the minutes of the Committee meeting held on 1 August 2016, be confirmed as a true and correct record. 5 September 2016 -

18 May 2016 Rangitata Island Dairy Limited Attn To
18 May 2016 Rangitata Island Dairy Limited Attn To: Simon Johnson 227 Wallace Road Rangitata RD 26 Geraldine 7986 Dear Sir/Madam NOTICE OF RESOURCE CONSENT DECISION(S) RECORD NO: CRC167754 NAME: Rangitata Island Dairy Limited The decision of Environment Canterbury is to grant your application(s) on the terms and conditions specified in the attached resource consent document(s). Your resource consent(s) commences from the date of this letter advising you of the decision. The reasons for the decision are: 1. The proposed activity does not conflict with the purpose of the RM Act given the conditions. For some activities a report is prepared, with officer recommendations, to provide information to the decision makers. If you require a copy of the report please contact our Customer Services section. If you do not agree with the consent authority decision, you may object to the whole or any part. Notice of any objection must be in writing and lodged with Environment Canterbury within 15 working days of receipt of this decision. Alternatively you may appeal to the Environment Court, PO Box 2069, Christchurch. The notice of appeal must be lodged with the Court within 15 working days of receipt of this decision, with a copy forwarded to Environment Canterbury within the same timeframe. If you appeal this decision, the commencement date will then be the date on which the decision on the appeal is determined. If you are in any doubt about the correct procedures, you should seek legal advice. Environment Canterbury takes every measure to improve both applications and processes, and we appreciate your feedback as an important component in ensuring this occurs. -

Timaru District OVG Supplementary Information
DECEMBER: DECEMBER: Christmas on the Bay the on Christmas be amazing! be Mountain bike at Centennial Park Centennial at bike Mountain Get your selfie on at our street art sites art street our at on selfie your Get Sample locally brewed craft beer craft brewed locally Sample GERALDINE | PEEL FOREST | MESOPOTAMIA | FOREST PEEL | GERALDINE to turn this into a sculpture trail - it’s going to to going it’s - trail sculpture a into this turn to machinery museums machinery Swim and picnic in our scenic gorges scenic our in picnic and Swim DECEMBER: in Timaru in TIMARU | TEMUKA | PLEASANT POINT PLEASANT | TEMUKA | TIMARU Timaru Festival of Roses of Festival Timaru A project is underway underway is project A . area shopping main the Explore five specialist vintage vehicle and and vehicle vintage specialist five Explore Pick up some cute cupcakes from Millie Rose Rose Millie from cupcakes cute some up Pick loop takes in a riverside walk, native forest, and and forest, native walk, riverside a in takes loop Visit friendly farmyard animals farmyard friendly Visit DECEMBER: DECEMBER: Caroline Bay Carnival Bay Caroline This This . foot on is Geraldine experience to ways with Te Ana Māori Rock Art Centre and Tours and Centre Art Rock Māori Ana Te with Strawberry Divine in Pleasant Point Pleasant in Divine Strawberry Riverside Walkway, Geraldine. Geraldine. Walkway, Riverside One of the best best the of One Go native bat spotting bat native Go Discover the ancient art galleries of Aotearoa Aotearoa of galleries art ancient the Discover NOVEMBER: NOVEMBER: Get a legendary real fruit ice cream at at cream ice fruit real legendary a Get Get to the Point Point the to Get . -

Consider Your Options When the Time Comes to Make Decisions Regarding the Final Care and Celebration of Life for Yourself Or a Loved One, Reach out to Us
Visit Temuka Telegraph’s Facebook page October 2019 | The Temuka Telegraph | 1 COMPLIMENTARY MONTHLY PUBLICATION YOUR NEWS | YOUR COMMUNITY OCTOBER 2019 Temuka Primary School Spring Fair - September 7th O’Reilly vs Falloon - inaugural ‘race’ around the schools new bike track photograph: Rose Austen-Falloon Consider Your Options When the time comes to make decisions regarding the final care and celebration of life for yourself or a loved one, reach out to us. Funeral Services 0800 686 2148 [email protected] www.aoraki.kiwi.nz SOUTH CANTERBURY 2 | The Temuka Telegraph | October 2019 www.temukatelegraph.co.nz from our MP’s desk... Where’s the BEEF ? We are in Temuka every Tuesday Andrew Falloon, Rangitata MP Call Chris Jones or Quentin Hix About four years ago, well before I became an MP, I found myself (Temuka locals who understand the community) dining at a table that included filmmaker James Cameron. 0800 QUENTIN / 03 687 9010 Although well known for his blockbusters Titanic and the Terminator, the conversation eventually swung around to farming. www.quentinhix.co.nz Explaining his love for the Wairarapa where he now spends much of his time, he talked of the several thousand hectares he was in the process of converting to fruit, nuts and vegetables. In the years since Mr Cameron has gone further. From a perfectly Central Podiatry Limited Central Podiatry Limited reasonable position of advancing the vegan cause on his own property, he’s now looking to enforce it nationwide. At a conference organised by the Government in Taranaki NathanNathan Dickson/ Dickson/ Louise Aubrey Louise recently, Aubrey Cameron likened our love of meat and dairy to an “illness or condition that has to be treated,” and called on farmers to shift to plant-based protein and milk.