Kidney Functions: -Osmoregulation -Blood Volume Regulation -Maintain
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

ZOOLOGY Animal Physiology Osmoregulation in Aquatic
Paper : 06 Animal Physiology Module : 27 Osmoregulation in Aquatic Vertebrates Development Team Principal Investigator: Prof. Neeta Sehgal Department of Zoology, University of Delhi Co-Principal Investigator: Prof. D.K. Singh Department of Zoology, University of Delhi Paper Coordinator: Prof. Rakesh Kumar Seth Department of Zoology, University of Delhi Content Writer: Dr Haren Ram Chiary and Dr. Kapinder Kirori Mal College, University of Delhi Content Reviewer: Prof. Neeta Sehgal Department of Zoology, University of Delhi 1 Animal Physiology ZOOLOGY Osmoregulation in Aquatic Vertebrates Description of Module Subject Name ZOOLOGY Paper Name Zool 006: Animal Physiology Module Name/Title Osmoregulation Module Id M27:Osmoregulation in Aquatic Vertebrates Keywords Osmoregulation, Active ionic regulation, Osmoconformers, Osmoregulators, stenohaline, Hyperosmotic, hyposmotic, catadromic, anadromic, teleost fish Contents 1. Learning Objective 2. Introduction 3. Cyclostomes a. Lampreys b. Hagfish 4. Elasmobranches 4.1. Marine elasmobranches 4.2. Fresh-water elasmobranches 5. The Coelacanth 6. Teleost fish 6.1. Marine Teleost 6.2. Fresh-water Teleost 7. Catadromic and anadromic fish 8. Amphibians 8.1. Fresh-water amphibians 8.2. Salt-water frog 9. Summary 2 Animal Physiology ZOOLOGY Osmoregulation in Aquatic Vertebrates 1. Learning Outcomes After studying this module, you shall be able to • Learn about the major strategies adopted by different aquatic vertebrates. • Understand the osmoregulation in cyclostomes: Lamprey and Hagfish • Understand the mechanisms adopted by sharks and rays for osmotic regulation • Learn about the strategies to overcome water loss and excess salt concentration in teleosts (marine and freshwater) • Analyse the mechanisms for osmoregulation in catadromic and anadromic fish • Understand the osmotic regulation in amphibians (fresh-water and in crab-eating frog, a salt water frog). -

Excretory Products and Their Elimination
290 BIOLOGY CHAPTER 19 EXCRETORY PRODUCTS AND THEIR ELIMINATION 19.1 Human Animals accumulate ammonia, urea, uric acid, carbon dioxide, water Excretory and ions like Na+, K+, Cl–, phosphate, sulphate, etc., either by metabolic System activities or by other means like excess ingestion. These substances have to be removed totally or partially. In this chapter, you will learn the 19.2 Urine Formation mechanisms of elimination of these substances with special emphasis on 19.3 Function of the common nitrogenous wastes. Ammonia, urea and uric acid are the major Tubules forms of nitrogenous wastes excreted by the animals. Ammonia is the most toxic form and requires large amount of water for its elimination, 19.4 Mechanism of whereas uric acid, being the least toxic, can be removed with a minimum Concentration of loss of water. the Filtrate The process of excreting ammonia is Ammonotelism. Many bony fishes, 19.5 Regulation of aquatic amphibians and aquatic insects are ammonotelic in nature. Kidney Function Ammonia, as it is readily soluble, is generally excreted by diffusion across 19.6 Micturition body surfaces or through gill surfaces (in fish) as ammonium ions. Kidneys do not play any significant role in its removal. Terrestrial adaptation 19.7 Role of other necessitated the production of lesser toxic nitrogenous wastes like urea Organs in and uric acid for conservation of water. Mammals, many terrestrial Excretion amphibians and marine fishes mainly excrete urea and are called ureotelic 19.8 Disorders of the animals. Ammonia produced by metabolism is converted into urea in the Excretory liver of these animals and released into the blood which is filtered and System excreted out by the kidneys. -

Claudins in the Renal Collecting Duct
International Journal of Molecular Sciences Review Claudins in the Renal Collecting Duct Janna Leiz 1,2 and Kai M. Schmidt-Ott 1,2,3,* 1 Department of Nephrology and Intensive Care Medicine, Charité-Universitätsmedizin Berlin, 12203 Berlin, Germany; [email protected] 2 Molecular and Translational Kidney Research, Max-Delbrück-Center for Molecular Medicine in the Helmholtz Association (MDC), 13125 Berlin, Germany 3 Berlin Institute of Health (BIH), 10178 Berlin, Germany * Correspondence: [email protected]; Tel.: +49-(0)30-450614671 Received: 22 October 2019; Accepted: 20 December 2019; Published: 28 December 2019 Abstract: The renal collecting duct fine-tunes urinary composition, and thereby, coordinates key physiological processes, such as volume/blood pressure regulation, electrolyte-free water reabsorption, and acid-base homeostasis. The collecting duct epithelium is comprised of a tight epithelial barrier resulting in a strict separation of intraluminal urine and the interstitium. Tight junctions are key players in enforcing this barrier and in regulating paracellular transport of solutes across the epithelium. The features of tight junctions across different epithelia are strongly determined by their molecular composition. Claudins are particularly important structural components of tight junctions because they confer barrier and transport properties. In the collecting duct, a specific set of claudins (Cldn-3, Cldn-4, Cldn-7, Cldn-8) is expressed, and each of these claudins has been implicated in mediating aspects of the specific properties of its tight junction. The functional disruption of individual claudins or of the overall barrier function results in defects of blood pressure and water homeostasis. In this concise review, we provide an overview of the current knowledge on the role of the collecting duct epithelial barrier and of claudins in collecting duct function and pathophysiology. -

The Osmotic Adjustment of Marine Microalgae
The osmotic adjustment of marine microalgae and the complex role osmolytes play in this process Thesis of Liz Kendall Supervisor Dr. Gieskes Department of Marine Biology, The University of Groningen April 1996 D5O Summary: Algae inhabit a wide variety of both marine and freshwater habitats. These habitats differ in regard to various factors such as chemical composition, the organisms that live there, the light which may radiate into that particular area, the temperature of the sites depending on where the environment is located, just to name a few. One factor that varies from environment to environment is the salinity. This paper will look at the mechanisms utilized by marine algae to cope with the changes in salinity content in their habitats and most importantly how they use different osmolytes to carry out this process. Marine algae "osmotically adjust" themselves to external salinity changes, in a biphasic maimer. Firstly, this includes changes in turgor pressure or large internal water fluxes in response to osmotic gradients. Secondly. an internally regulated osmotic adjustment occurs with the use of both inorganic and organic osmolytes. Compatible solutes are ions and molecules used by man organisms to osmotically adjust and they play a double role in the process of osmotic adjustment. They act as osmolytes and also protect the cellular enzymatic activities under salinity stress. They are called 'compatible solutes" because they protect the cellular enzymatic activity. The main compatible solutes are polyols (including amino acids, carbohydrates and sugars). quaternary ammonium derivatives or tertiary sulphonium compounds. Certain species and taxonomic classes use specific compatible solutes and some even use combinations of them. -

Morphology of the Kidney in a Nectarivorous Bird, the Anna's Hummingbird Calypte Anna
J. Zool., Lond. (1998) 244, 175±184 # 1998 The Zoological Society of London Printed in the United Kingdom Morphology of the kidney in a nectarivorous bird, the Anna's hummingbird Calypte anna G. Casotti1*, C. A. Beuchat2 and E. J. Braun3 1 Department of Biology, West Chester University, West Chester, PA, 19383, U.S.A. 2 Department of Biology, San Diego State University, San Diego, CA, 92182, U.S.A. 3 Department of Physiology, Arizona Health Sciences Center, University of Arizona, P.O. Box 245051, Tucson, AZ, 85724±5051, U.S.A. (Accepted 21 April 1997) Abstract The kidneys of Anna's hummingbird (Calypte anna) differ in several signi®cant ways from those of other birds that have been examined. The kidneys of this nectarivore contain very little medullary tissue; 90% of the total volume of the kidneys is cortical tissue, with medulla accounting for only an additional 2%. More than 99% of the nephrons are the so-called `reptilian type', which lack the loop of Henle. The few looped (`mammalian type') nephrons are incorporated into only a few medullary cones per kidney. The loopless nephrons are similar to those of other birds. However, the looped nephrons differ in that they lack the thin descending limb of the loop of Henle, which is found in other birds and is thought to play an important role in the countercurrent multiplier system in the avian kidney. Instead, the cells of the nephron segment following the pars recta of the proximal tubule resemble those of the thick ascending limb, with the large populations of mitochondria that are typical of transporting epithelia and no reduction in cell height. -

Birds in Marine and Saline Environments: Living in Dry Habitats
Revista Chilena de Historia Natural 73: 401-410, 2000 Birds in marine and saline environments: living in dry habitats Aves en ambientes marinos y salinos: viviendo en habitats secos PABLOSABAT Departamento de Ciencias Ecologicas, Facultad de Ciencias, Universidad de Chile, Casilla 653, Santiago, Chile, e-mail: psabat@ abello.dic. uchile.cl ABSTRACT For birds, saline environments such as maritime and salt marsh habitats are essentially dry habitats. When birds drink saline water or consume salt-loaded preys, the osmolarity of their body fluids increases. In order to maintain the osmotic equilibrium, they have to eliminate the excess of electrolytes ingested with preys or water. Marine birds use salt glands, which produce excretion solutions more concentrated than seawater to eliminate excess salt. The physiology and phenotypic plasticity of nasal glands appears to be correlated with the ecological features of species. Birds can also minimize osmotic stress by choosing hypo-osmotic preys, preys with reduced water content, and/or by decreasing salt intake. Although the kidney of birds is clearly less efficiently in its capacity to concentrate the urine than that of mammals, there are interspecific differences in renal structure and physiology that may be correlated with the birds ecological habits, and hence to represent adaptive mechanism to prevent water loss. The kidney may be especially important in taxa that lack active salt gland, such as passerines. Passerines, which are supposed to have limited ability to use saline habitats, include several marine and salt-marsh species. In this review I show that the interaction of the kidney and rectum in osmoregulatory physiology, coupled with selective feeding behavior play a major role in the maintenance of water and salt balance of passerines living in salty environments. -

Energetic Aspects of Osmoregulation in Fish
ENERGETIC ASPECTS OF OSMOREGULATION IN FISH by JOHN DAVID MORGAN B.Sc, The University of British Columbia, 1979 M.Sc, The University of British Columbia, 1991 A THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY in THE FACULTY OF GRADUATE STUDIES (Department of Animal Science) We accept this thesis as conforming to the required standard THE UNIVERSITY OF BRITISH COLUMBIA September 1997 © John David Morgan In presenting this thesis in partial fulfilment of the requirements for an advanced degree at the University of British Columbia, I agree that the Library shall make it freely available for reference and study. I further agree that permission for extensive copying of this thesis for scholarly purposes may be granted by the head of my department or by his or her representatives. It is understood that copying or publication of this thesis for financial gain shall not be allowed without my written permission. v , Department of f\ ^v. »v^A. Se,ve^cg_, The University of British Columbia Vancouver, Canada Date Se-pA^W KVI DE-6 (2/88) 11 ABSTRACT The energetic aspects of osmoregulation in several species of fish were examined, using an experimental approach on both a whole-animal and tissue level. The first series of experiments examined the metabolic response of temperate and tropical fish species to acute and gradual salinity change, using whole-animal oxygen consumption rates and gill Na+,K+-ATPase activity as indicators of osmoregulatory energetics. Juvenile dolphin fish (Coryphaena hippurus) were exposed for 24 h to a reduced water salinity (34 to 20 ppt). -

Factfile: Gcse Biology: Unit 1.6
FACTFILE: GCSE BIOLOGY: UNIT 1.6 Homeostasis Learning outcomes Glucose concentration Glucose is needed for respiration to produce energy Students should be able to: for cells, if a cell cannot produce enough energy • 1.6.9 explain the importance of maintaining a it will not be able to carry out certain chemical constant internal environment for the proper reactions it needs to survive. The opposite problem, functioning of cells and enzymes in response having too much glucose, can also cause damage to internal and external change, limited to to cells. controlling blood glucose concentration and osmoregulation. Water concentration All the chemical reactions in cells take place in water. The reactants must be dissolved in water, Why is it important to maintain a therefore the water concentration of cells and the constant internal environment? surrounding fluid is vital. The human body is made up of billions of cells working together to maintain the whole body. Although cells may perform different functions in different parts of the body they all have very similar needs to survive. Cells are very sensitive to changes in their environment so the conditions inside the body must stay within a very narrow range for cells to function as efficiently as possible. Changes in both the internal and external environments are a constant for living things. The body responds to these changes by a process called homeostasis. Library © SciencePhoto Homeostasis is the process of maintaining Dissolved substances such as salts, affect the a stable internal environment for the proper balance of these fluids and so must also be functioning of cells, in response to internal and controlled. -

The Multifunctional Gut of Fish
THE MULTIFUNCTIONAL GUT OF FISH 1 Zebrafish: Volume 30 Copyright r 2010 Elsevier Inc. All rights reserved FISH PHYSIOLOGY DOI: This is Volume 30 in the FISH PHYSIOLOGY series Edited by Anthony P. Farrell and Colin J. Brauner Honorary Editors: William S. Hoar and David J. Randall A complete list of books in this series appears at the end of the volume THE MULTIFUNCTIONAL GUT OF FISH Edited by MARTIN GROSELL Marine Biology and Fisheries Department University of Miami-RSMAS Miami, Florida, USA ANTHONY P. FARRELL Faculty of Agricultural Sciences The University of British Columbia Vancouver, British Columbia Canada COLIN J. BRAUNER Department of Zoology The University of British Columbia Vancouver, British Columbia Canada AMSTERDAM • BOSTON • HEIDELBERG • LONDON • OXFORD NEW YORK • PARIS • SAN DIEGO • SAN FRANCISCO SINGAPORE • SYDNEY • TOKYO Academic Press is an imprint of Elsevier Academic Press is an imprint of Elsevier 32 Jamestown Road, London NW1 7BY, UK 30 Corporate Drive, Suite 400, Burlington, MA 01803, USA 525 B Street, Suite 1800, San Diego, CA 92101-4495, USA First edition 2011 Copyright r 2011 Elsevier Inc. All rights reserved No part of this publication may be reproduced, stored in a retrieval system or transmitted in any form or by any means electronic, mechanical, photocopying, recording or otherwise without the prior written permission of the publisher Permissions may be sought directly from Elsevier’s Science & Technology Rights Department in Oxford, UK: phone (þ44) (0) 1865 843830; fax (þ44) (0) 1865 853333; email: [email protected]. Alternatively, visit the Science and Technology Books website at www.elsevierdirect.com/rights for further information Notice No responsibility is assumed by the publisher for any injury and/or damage to persons or property as a matter of products liability, negligence or otherwise, or from any use or operation of any methods, products, instructions or ideas contained in the material herein. -

Salinity Stress Responses and Adaptation Mechanisms in Eukaryotic Green Microalgae
cells Review Salinity Stress Responses and Adaptation Mechanisms in Eukaryotic Green Microalgae Prateek Shetty 1, Margaret Mukami Gitau 1 and Gergely Maróti 1,2,* 1 Institute of Plant Biology, Hungarian Academy of Sciences, Biological Research Centre, 6726 Szeged, Hungary; [email protected] (P.S.); [email protected] (M.M.G.) 2 Faculty of Water Sciences, National University of Public Service, 6500 Baja, Hungary * Correspondence: [email protected]; Tel.: +36-308-270455 Received: 1 October 2019; Accepted: 12 December 2019; Published: 17 December 2019 Abstract: High salinity is a challenging environmental stress for organisms to overcome. Unicellular photosynthetic microalgae are especially vulnerable as they have to grapple not only with ionic imbalance and osmotic stress but also with the generated reactive oxygen species (ROS) interfering with photosynthesis. This review attempts to compare and contrast mechanisms that algae, particularly the eukaryotic Chlamydomonas microalgae, exhibit in order to immediately respond to harsh conditions caused by high salinity. The review also collates adaptation mechanisms of freshwater algae strains under persistent high salt conditions. Understanding both short-term and long-term algal responses to high salinity is integral to further fundamental research in algal biology and biotechnology. Keywords: high salt stress; green algae; adaptation; transcriptome; salinity; Chlamydomonas 1. Introduction Algae refer to a broad group of micro- and macroorganisms capable of oxygenic photosynthesis, yet show striking differences compared to land plants. The traditional and common definition of algae includes prokaryotic cyanobacteria and eukaryotic algae belonging to highly different phylogenetic clades. Eukaryotic microalgae are polyphyletic in origin and have an extensive ecological niche and evolutionary history. -

The Hormonal Control of Osmoregulation in Teleost Fish
Provided for non-commercial research and educational use. Not for reproduction, distribution or commercial use. This article was originally published in Encyclopedia of Fish Physiology: From Genome to Environment, published by Elsevier, and the attached copy is provided by Elsevier for the author’s benefit and for the benefit of the author’s institution, for non-commercial research and educational use including without limitation use in instruction at your institution, sending it to specific colleagues who you know, and providing a copy to your institution’s administrator. All other uses, reproduction and distribution, including without limitation commercial reprints, selling or licensing copies or access, or posting on open internet sites, your personal or institution’s website or repository, are prohibited. For exceptions, permission may be sought for such use through Elsevier’s permissions site at: http://www.elsevier.com/locate/permissionusematerial McCormick S.D. (2011) The Hormonal Control of Osmoregulation in Teleost Fish. In: Farrell A.P., (ed.), Encyclopedia of Fish Physiology: From Genome to Environment, volume 2, pp. 1466–1473. San Diego: Academic Press. ª 2011 Elsevier Inc. All rights reserved. Author's personal copy HORMONAL CONTROLS Hormonal Control of Metabolism and Ionic Regulation Contents The Hormonal Control of Osmoregulation in Teleost Fish Corticosteroids The Hormonal Control of Osmoregulation in Teleost Fish S D McCormick, USGS, Conte Anadromous Fish Research Center, Turners Falls, MA, USA Published by Elsevier Inc. Introduction: Salt and Water Balance in Fish Thyroid Hormones Cortisol The Special Case of Anadromy Prolactin Summary Growth Hormone and IGF-I Further Reading Glossary Insulin-like growth factor-I (IGF-I) A protein Adrenocorticotrophic hormone (ACTH) A protein hormone produced in the liver in response to growth hormone produced in the pituitary that causes release of hormone and acting on growth and ion regulation. -
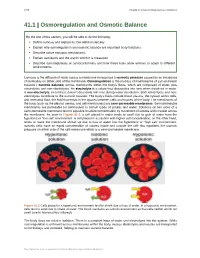
Osmoregulation and Osmotic Balance
1278 Chapter 41 | Osmotic Regulation and Excretion 41.1 | Osmoregulation and Osmotic Balance By the end of this section, you will be able to do the following: • Define osmosis and explain its role within molecules • Explain why osmoregulation and osmotic balance are important body functions • Describe active transport mechanisms • Explain osmolarity and the way in which it is measured • Describe osmoregulators or osmoconformers and how these tools allow animals to adapt to different environments Osmosis is the diffusion of water across a membrane in response to osmotic pressure caused by an imbalance of molecules on either side of the membrane. Osmoregulation is the process of maintenance of salt and water balance ( osmotic balance) across membranes within the body’s fluids, which are composed of water, plus electrolytes and non-electrolytes. An electrolyte is a solute that dissociates into ions when dissolved in water. A non-electrolyte, in contrast, doesn’t dissociate into ions during water dissolution. Both electrolytes and non- electrolytes contribute to the osmotic balance. The body’s fluids include blood plasma, the cytosol within cells, and interstitial fluid, the fluid that exists in the spaces between cells and tissues of the body. The membranes of the body (such as the pleural, serous, and cell membranes) are semi-permeable membranes. Semi-permeable membranes are permeable (or permissive) to certain types of solutes and water. Solutions on two sides of a semi-permeable membrane tend to equalize in solute concentration by movement of solutes and/or water across the membrane. As seen in Figure 41.2, a cell placed in water tends to swell due to gain of water from the hypotonic or “low salt” environment.