Oral Lesions in Neonates Review Article
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Glossary for Narrative Writing
Periodontal Assessment and Treatment Planning Gingival description Color: o pink o erythematous o cyanotic o racial pigmentation o metallic pigmentation o uniformity Contour: o recession o clefts o enlarged papillae o cratered papillae o blunted papillae o highly rolled o bulbous o knife-edged o scalloped o stippled Consistency: o firm o edematous o hyperplastic o fibrotic Band of gingiva: o amount o quality o location o treatability Bleeding tendency: o sulcus base, lining o gingival margins Suppuration Sinus tract formation Pocket depths Pseudopockets Frena Pain Other pathology Dental Description Defective restorations: o overhangs o open contacts o poor contours Fractured cusps 1 ww.links2success.biz [email protected] 914-303-6464 Caries Deposits: o Type . plaque . calculus . stain . matera alba o Location . supragingival . subgingival o Severity . mild . moderate . severe Wear facets Percussion sensitivity Tooth vitality Attrition, erosion, abrasion Occlusal plane level Occlusion findings Furcations Mobility Fremitus Radiographic findings Film dates Crown:root ratio Amount of bone loss o horizontal; vertical o localized; generalized Root length and shape Overhangs Bulbous crowns Fenestrations Dehiscences Tooth resorption Retained root tips Impacted teeth Root proximities Tilted teeth Radiolucencies/opacities Etiologic factors Local: o plaque o calculus o overhangs 2 ww.links2success.biz [email protected] 914-303-6464 o orthodontic apparatus o open margins o open contacts o improper -

Oral Diagnosis: the Clinician's Guide
Wright An imprint of Elsevier Science Limited Robert Stevenson House, 1-3 Baxter's Place, Leith Walk, Edinburgh EH I 3AF First published :WOO Reprinted 2002. 238 7X69. fax: (+ 1) 215 238 2239, e-mail: [email protected]. You may also complete your request on-line via the Elsevier Science homepage (http://www.elsevier.com). by selecting'Customer Support' and then 'Obtaining Permissions·. British Library Cataloguing in Publication Data A catalogue record for this book is available from the British Library Library of Congress Cataloging in Publication Data A catalog record for this book is available from the Library of Congress ISBN 0 7236 1040 I _ your source for books. journals and multimedia in the health sciences www.elsevierhealth.com Composition by Scribe Design, Gillingham, Kent Printed and bound in China Contents Preface vii Acknowledgements ix 1 The challenge of diagnosis 1 2 The history 4 3 Examination 11 4 Diagnostic tests 33 5 Pain of dental origin 71 6 Pain of non-dental origin 99 7 Trauma 124 8 Infection 140 9 Cysts 160 10 Ulcers 185 11 White patches 210 12 Bumps, lumps and swellings 226 13 Oral changes in systemic disease 263 14 Oral consequences of medication 290 Index 299 Preface The foundation of any form of successful treatment is accurate diagnosis. Though scientifically based, dentistry is also an art. This is evident in the provision of operative dental care and also in the diagnosis of oral and dental diseases. While diagnostic skills will be developed and enhanced by experience, it is essential that every prospective dentist is taught how to develop a structured and comprehensive approach to oral diagnosis. -

A Single Case Report of Granular Cell Tumor of the Tongue Successfully Treated Through 445 Nm Diode Laser
healthcare Case Report A Single Case Report of Granular Cell Tumor of the Tongue Successfully Treated through 445 nm Diode Laser Maria Vittoria Viani 1,*, Luigi Corcione 1, Chiara Di Blasio 2, Ronell Bologna-Molina 3 , Paolo Vescovi 1 and Marco Meleti 1 1 Department of Medicine and Surgery, University of Parma, 43126 Parma, Italy; [email protected] (L.C.); [email protected] (P.V.); [email protected] (M.M.) 2 Private practice, Centro Medico Di Blasio, 43121 Parma; Italy; [email protected] 3 Faculty of Dentistry, University of the Republic, 14600 Montevideo, Uruguay; [email protected] * Correspondence: [email protected] Received: 10 June 2020; Accepted: 11 August 2020; Published: 13 August 2020 Abstract: Oral granular cell tumor (GCT) is a relatively rare, benign lesion that can easily be misdiagnosed. Particularly, the presence of pseudoepitheliomatous hyperplasia might, in some cases, lead to the hypothesis of squamous cell carcinoma. Surgical excision is the treatment of choice. Recurrence has been reported in up to 15% of cases treated with conventional surgery. Here, we reported a case of GCT of the tongue in a young female patient, which was successfully treated through 445 nm diode laser excision. Laser surgery might reduce bleeding and postoperative pain and may be associated with more rapid healing. Particularly, the vaporization effect on remnant tissues could eliminate GCT cells on the surgical bed, thus hypothetically leading to a lower rate of recurrence. In the present case, complete healing occurred in 1 week, and no recurrence was observed after 6 months. Laser surgery also allows the possibility to obtain second intention healing. -
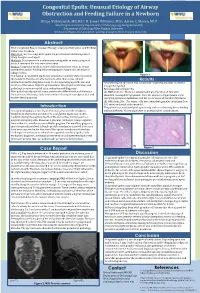
Congenital Epulis: Unusual Etiology of Airway Obstruction and Feeding Failure in a Newborn Shilpa Vishwanath, MD,MS1; H
Congenital Epulis: Unusual Etiology of Airway Obstruction and Feeding Failure in a Newborn Shilpa Vishwanath, MD,MS1; H. James Williams, MD2; Aaron C. Mason, MD3 1West Virginia University Department of Otolaryngology, Morgantown WV; 2Department of Pathology, West Virginia University 3Division of Plastic, Reconstructive, and Hand Surgery, West Virginia University Abstract Title: Congenital Epulis: Unusual Etiology of Airway Obstruction and Feeding Failure in a Newborn Objectives: Review congenital epulis; Its presentation and management. Study Design: Case Report Methods: Description of a newborn presenting with an obstructing oral mass. A review of the literature is included. Results: Congenital epulis is a rare oral lesion that may result in airway obstruction and/or feeding failure bringing the mass to the attention of subspecialists. Conclusion: A congenital epulis may present as a solitary alveolar mass in Figure 1 the newborn. Females are affected more often than males. Airway Results obstruction and feeding failure may evolve depending upon the size and The pathological specimen was a maxillary congenital granular cell tumor location of the lesion. Physical examination, radiographic evaluation, and (congenital epulis). pathologic review are useful in its evaluation and diagnosis. Pathology slides (Figure 3): Histopathologically, special stains assist in the differentiation of the lesion (A) H&E stain, 4x: There is a subepithelial proliferation of cells with from other solid tumors. Early intervention relieves airway obstruction and abundant eosinophilic cytoplasm. Note the absence of hyperplasia of the enables feeding success. overlying squamous epithelium and the prominence of vascular structures. (B) H&E stain, 20x: The tumor cells have abundant granular cytoplasm (low N/C ratio) and small uniform nuclei. -

Treatments for Ankyloglossia and Ankyloglossia with Concomitant Lip-Tie Comparative Effectiveness Review Number 149
Comparative Effectiveness Review Number 149 Treatments for Ankyloglossia and Ankyloglossia With Concomitant Lip-Tie Comparative Effectiveness Review Number 149 Treatments for Ankyloglossia and Ankyloglossia With Concomitant Lip-Tie Prepared for: Agency for Healthcare Research and Quality U.S. Department of Health and Human Services 540 Gaither Road Rockville, MD 20850 www.ahrq.gov Contract No. 290-2012-00009-I Prepared by: Vanderbilt Evidence-based Practice Center Nashville, TN Investigators: David O. Francis, M.D., M.S. Sivakumar Chinnadurai, M.D., M.P.H. Anna Morad, M.D. Richard A. Epstein, Ph.D., M.P.H. Sahar Kohanim, M.D. Shanthi Krishnaswami, M.B.B.S., M.P.H. Nila A. Sathe, M.A., M.L.I.S. Melissa L. McPheeters, Ph.D., M.P.H. AHRQ Publication No. 15-EHC011-EF May 2015 This report is based on research conducted by the Vanderbilt Evidence-based Practice Center (EPC) under contract to the Agency for Healthcare Research and Quality (AHRQ), Rockville, MD (Contract No. 290-2012-00009-I). The findings and conclusions in this document are those of the authors, who are responsible for its contents; the findings and conclusions do not necessarily represent the views of AHRQ. Therefore, no statement in this report should be construed as an official position of AHRQ or of the U.S. Department of Health and Human Services. The information in this report is intended to help health care decisionmakers—patients and clinicians, health system leaders, and policymakers, among others—make well-informed decisions and thereby improve the quality of health care services. This report is not intended to be a substitute for the application of clinical judgment. -

Pigmented Villonodular Synovitis of the Temporomandibular Joint: a Case Report and the Literature Review
1314 Cai et al. Case Report TMJ Disorders J. Cai1, Z. Cai1, Y. Gao2 1Department of Oral and Maxillofacial Pigmented villonodular synovitis 2 Surgery, Beijing, China; Department of Oral Pathology, Peking University School & of the temporomandibular joint: Hospital of Stomatology, Beijing, China a case report and the literature review J. Cai, Z. Cai, Y. Gao: Pigmented villonodular synovitis of the temporomandibular joint: a case report and the literature review. Int. J. Oral Maxillofac. Surg. 2011; 40: 1314–1322. # 2011 International Association of Oral and Maxillofacial Surgeons. Published by Elsevier Ltd. All rights reserved. Abstract. Pigmented villonodular synovitis (PVNS) is an uncommon benign proliferative disorder of synovium that may involve joints, tendon sheaths, and bursae. It most often affects the knees, and less frequently involves other joints. It presents in the temporomandibular joints (TMJs) extremely rarely. The authors report an elderly female patient with PVNS of the TMJ with skull base extension, who had traumatic history in the same site. It was diagnosed through core-needle Keywords: pigmented villonodular synovitis (PVNS); synovitis; temporomandibular joint biopsy, which was not documented in the literature. Radical excision and follow-up (TMJ). for 7–8 years was recommended because of the reported malignant transformation and high recurrence rate. This case and previously reported cases in the literature are Accepted for publication 2 March 2011 reviewed and discussed. Available online 6 April 2011 The term pigmented villonodular synovi- were reported in detail (Table 1). The visits and mouth-opening for a long time tis (PVNS) was introduced by JAFFE et al. authors present an additional case of during the operation. -

Salivary Gland Pathology 25.Pdf
k Index 461 Mechanoreceptors, 15 patient history, 284 Melanoma pleomorphic adenoma, 286–290 desmoplastic subtype, 63 polymorphous low-grade adenocarcinoma, 174–175, 306, histopathology, 381 309, 312 lower lip, 380–381 primary lymphomas, 368 metastases, 62–63, 189 radiation therapy, 328 nodular, 379 sites of, 285 Merkel cell tumors, metastases, 62, 63, 375 staging, 193–196 Mesenchymal-epithelial transition (MET), 214 Mixed tumor. See Pleomorphic adenoma(s) Mesenchymal neoplasms, 188 Modified Blair incision, 238, 239 Mesenchymal salivary gland tumors Monomorphic adenoma, 167–169, 290 lymphatic malformations, 398, 400 Monomorphic clear cell tumor, 182 neural tumors, 398, 401, 402 Motion artifacts, 21–22 vascular tumors, 397–398, 397–399 Mouth Messenger ribonucleic acid (mRNA), 208, 209, 209 dry, 141 Metal deposits, brain, 24 ranula, 98, 99, 100 Metallic implants, 21, 22 MRI. See Magnetic resonance imaging (MRI) Metalloproteinases, 142 MRS. See Magnetic resonance spectroscopy (MRS) Metastases, 189 Mucocele, 97, 99, 114, 412 diagnostic imaging, 62–64, 63 Mucoepidermoid carcinoma (MEC), 170–172, 214, 389, distant. See Distant metastases 390, 396, 404 regional. See Regional metastases ADC values, 27 skip, 270 biomarkers, 190, 263 Metastasizing mixed tumor, 182, 289 buccal mucosa, 294 Metastasizing pleomorphic adenoma, 287 children, 297 Methicillin resistant S. aureus (MRSA), acute bacterial clear cell variant, 383 parotitis, 75, 78, 96 diagnostic imaging, 56, 57 k Microliths, 439 fixed to mandible, 277 k Middle ear, aberrant glands, 438 grading, -

Description Concept ID Synonyms Definition
Description Concept ID Synonyms Definition Category ABNORMALITIES OF TEETH 426390 Subcategory Cementum Defect 399115 Cementum aplasia 346218 Absence or paucity of cellular cementum (seen in hypophosphatasia) Cementum hypoplasia 180000 Hypocementosis Disturbance in structure of cementum, often seen in Juvenile periodontitis Florid cemento-osseous dysplasia 958771 Familial multiple cementoma; Florid osseous dysplasia Diffuse, multifocal cementosseous dysplasia Hypercementosis (Cementation 901056 Cementation hyperplasia; Cementosis; Cementum An idiopathic, non-neoplastic condition characterized by the excessive hyperplasia) hyperplasia buildup of normal cementum (calcified tissue) on the roots of one or more teeth Hypophosphatasia 976620 Hypophosphatasia mild; Phosphoethanol-aminuria Cementum defect; Autosomal recessive hereditary disease characterized by deficiency of alkaline phosphatase Odontohypophosphatasia 976622 Hypophosphatasia in which dental findings are the predominant manifestations of the disease Pulp sclerosis 179199 Dentin sclerosis Dentinal reaction to aging OR mild irritation Subcategory Dentin Defect 515523 Dentinogenesis imperfecta (Shell Teeth) 856459 Dentin, Hereditary Opalescent; Shell Teeth Dentin Defect; Autosomal dominant genetic disorder of tooth development Dentinogenesis Imperfecta - Shield I 977473 Dentin, Hereditary Opalescent; Shell Teeth Dentin Defect; Autosomal dominant genetic disorder of tooth development Dentinogenesis Imperfecta - Shield II 976722 Dentin, Hereditary Opalescent; Shell Teeth Dentin Defect; -

Subject Index
SUBJECT INDEX SUBJECT INDEX Abbe flap, vermilion submucosal pedicle, for upper lip Accurate platelet counts for platelet rich plasma, validation reconstruction (Special Section: Cancer), 17:1259– of hematology analyzer and preparation techniques 1262 for counting and (Scientific Foundation), 16:749– Abbé island flap (Original Articles), 18:766–768 759 Abducens nerve, microanatomic and endoscopic study Acellular cadaveric dermis, experimental study of (Scientific (Literature Scan), 19:546 Foundations), 18:551–558 Abortive subtype of frontoethmoidal encephalocele (Case Acellular human dermis (alloderm), exposed skull with, one- Report), 10:149–154 stage skin grafting of (Technical Experience), Abraxane, for treatment of metastatic breast cancer (Special 19:1660–1662 Editorial), 17:3–7 Acetylic resin, in conjunction with silicon for maxillofacial Abrikossoff’s tumor (Clinical Note), 12:78–81 rehabilitation (Brief Clinical Notes), 17:152–162 Abscess, brain, from cranio-orbital foreign body (Clinical Achondroplasia and Pfeiffer syndrome, genetic relationship Note), 7:311–314 between (Clinical Note), 9:477–480 Absent ear, bone-anchored implants for (Scientific Acoustic evoked potentials in neurophysiological Foundation), 19:744–747 evaluation in craniostenosis and craniofacial Absorbable. See also under Bioabsorbable stenosis, 8:286–289 Absorbable biomaterial in craniofacial skeleton fixation, Acquired orbital deformity, reconstruction of (Clinical 10:491 Note), 19:1092–1097 Absorbable fixation Acrocephalosyndactyly, 7:23–30, 8:279–283 for -

Oral Anomalies in the Neonate, by Race and Gender, in an Urban Setting Gerald W
PEDIATRICDENTISTRY/Copyright © 1990 by The AmericanAcademy of Pediatric Dentistry Volume 12, Number3 Oral anomalies in the neonate, by race and gender, in an urban setting Gerald W. Friend, DDS, MS Edward F. Harris, PhD Harry H. Mincer, DDS, MS, PhD Teresa L. Fong, DDS Kenneth R. Carruth, DDS, MS Abstract A broad range of developmental anomalies and morphol- median alveolar notch (Rao 1979; Jorgenson et al. 1982), ogic variants may occur in the oral cavity of the newborn. and leukoedema (Martin and Crump 1972; Jorgenson et Becausemany of these are transient (e.g.: palatal and alveolar al. 1982; Durocher et al. 1972; Shafer et al. 1983). Com- cysts, lymphangioma),self-correcting with age, conventional missural lip pits (Everett and Wescott 1964; Baker 1966; assessmentsof older children can yield significantly altered Jorgenson et al. 1982), ankyloglossia (McEnery and trait incidences. A total of 500 normal full-term newborns Gaines 1942; Mathewsonet al. 1966), natal teeth (Mas- (blacks and whites) wereassessed by standardizedcriteria for sler and Savara 1950; Anderson 1982; Kates et al. 1984; 11 oral conditions, in addition to collating data on maternal Leung 1986), and congenital epulis (Custer and Fust conditions (age, gravidity, tobaccoand alcohol use). Leukoe- 1952; Bhaskar and Akanum1955; Fuhr and Krogh 1972; demaand median alveolar notches were significantly more Welbury 1980) occur infrequently. Alveolar lym- commonin blacks, whereaspalatal cysts were 2.5 times as phangiomais a rare lesion which has been found only in likely to occur in whites. Ankyloglossia, three times as com- black infants (Levin et al. 1976; Kettle and Weaver1987). monin males, was the one trait to exhibit a significant predilection by gender. -

Congenital Epulis
Congenital Epulis Bernadette L. Koch, Charles Myer III, and John C. Egelhoff Summary: Congenital epulis of the newborn is a rare gingival surgeon. The infant was delivered without difficulty, with tumor that occurs along the alveolar ridge. We report the prena- Apgar score of 8/8/9 at 1, 5, and 10 minutes. The infant tal sonographic and postnatal MR imaging findings in an infant required no mechanical respiratory support, as the mass with maxillary and mandibular congenital epulides. did not obstruct the airway. After delivery, two separate Index terms: Temporomandibular joint, abnormalities and anom- pedunculated gingival masses were noted. One mass alies; Fetus, ultrasound arose from the anterior maxillary alveolar ridge and mea- sured 3 cm in greatest dimension; the second mass arose from the anterior mandibular alveolar ridge and measured The term congenital epulis of the newborn 2 cm (Fig 1B). The masses were well defined, smooth, and refers to a rare gingival tumor that most com- erythematous. The size and position of the masses caused monly occurs along the alveolar ridge of the superior deviation of the upper lip. Both nares were patent maxilla in newborn girls, usually without asso- with passage of an 8F catheter. The nose was flat and the ciated abnormalities of the teeth or additional nasal spine was absent, most likely as a result of deforma- congenital malformations. A case was de- tion from the gingival masses. Clinically, the osseous max- illary and mandibular ridges were thought to be normal. scribed by Neumann in 1871 (1). Since then, Postnatal MR imaging at 1 day of age revealed lobulat- multiple cases have been reported, primarily in ed masses that did not extend into the soft palate, floor of the pathologic, dental, and otolaryngologic lit- the mouth, nose, or cranium. -

Toma, 539 Acinic Cell Carcinoma Mucinous Adenocarcinoma Vs., 334
Cambridge University Press 978-0-521-87999-6 - Head and Neck Margaret Brandwein-Gensler Index More information INDEX acanthomatous/desmoplastic ameloblas- ameloblastomas benign neoplasia toma, 539 desmoplastic ameloblastoma, 539 juvenile nasopharyngeal angiofibroma, acinic cell carcinoma metastasizing ameloblastoma, 537 99–104 mucinous adenocarcinoma vs., 334–335 mural ameloblastoma, 533 salivary gland anlage tumor, 104–106 oncocytoma vs., 295–299 odonto-ameloblastoma, 551–553 benign peripheral nerve sheath tumors papillary cystic variant, vs. cystade- peripheral ameloblastoma, 534 (BPNST), 40–44 noma, 316–317 unicystic ameloblastoma, 532–533 benign sinonasal tract neoplasia, 28–48 salivary glands, 353–359 aneurysmal bone cyst (ABC), 584 benign peripheral nerve sheath tumor, adenocarcinoma not otherwise specified central GCRG vs., 590–591 40–44 (ANOS), 389–390 angiocentric T-cell lymphoma, 81 meningioma, 37–40 adenoid cystic carcinoma (ACC) angiomatoid/angioectatic polyps vs. JNAF, nasal glial heterotopia (NGH), 44–48 adenomatoid odontogenic tumor vs., 100–104 oncocytic Schneiderian papilloma 545 angiosarcoma vs. Kaposi’s sarcoma, 206 (OSP), 5, 33–36 basal cell adenocarcinoma vs., 372 antrochoanal polyp, 5–8 Schneiderian inverted papilloma, 28–32 basal cell adenoma vs., 293 apical periodontal cyst, 510–512 bisphosphonate osteonecrosis (BPP), canalicular adenoma vs., 294 arytenoid chondrosarcomas, 241 565–566 neuroendocrine carcinoma vs., 240–241 atrophic oral lichen planus, 126 blastomas of salivary glands, 319–325 atypical adenoma vs. parathyroid