The University of Oklahoma Graduate
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

II~I~~~[~I~Lll~~~~~II~Li[~~Lil ~R[[Lil~~1 U
, II~i~~~[~I~lll~~~~~II~li[~~lil ~r[[lil~~1 u. S. DEPARTMENT OF COMMERL_ 3 4589 000020379 Luther H. Hodges, Secretary WEATHER BUREAU Robert M. White, Chief . NATIONAL SEVERE STORMS LABORATORY REPORT NO. 23 Purposes and Programs of the· National Severe Storms Laboratory Norman, Oklahoma by Edwin Kessler Director . Washington, D. C. December 1964 . .. _._ ._ .... _--_.- ... - .. __.. - -_ .._ _.. _-- ---_ ... _. .. ... _.. _--- --_. _.... - ._ -.- -------, PURPOSES AND PROGRAM OF THE U.S. WEATHER BUREAU NATIONAL SEVERE STORMS LABORATORY NORMAN, OKLAHOMA Edwin Kessler Director, National Severe Storms Laboratory I. INTRODUCTION The needs for more accurate, timely, and meaningful weather information grow as production, transportation, and ccmmunication processes become more intricate and more weather-sensitive. Aviators, agriculturists, builders, shippers, and insurers are among those who increasingly depend on information concerning the present dnd forecast occur rences of storm hazards. Development of the national weather system to meet national needs efficiently depends on the appl ication of findings of basJc and applied meteorological research. Mopern technology has produced numerous research tools whose coordinated-use should contribute importantly to improved understanding of the meteorological processes associ ated with severe storm development, to more accurate weather analysis and forecasting, and to eventual beneficial modification or control of weather including severe storms:. The Nat-ional Severe Sto-rms Laborat9ry aims to extend our understanding of severe convective phenomena such as tornadoes, hailstorms, and heavy rains. For the study of thesephenome~a, modern weather radar is an indispensable tool, and considerable specialization in techniques of radar data display and processing, including synthesis of radar data with other kinds of information, is required. -

Special News Feature Americas Weather Warriors
special news feature Edwin Kessler Retires As Director of the National Severe Storms Laboratory Edwin Kessler retired in June from his position as di- ing system. He has taught as a visiting professor at MIT, rector of the National Severe Storms Laboratory in Nor- and he presented a series of seminars on precipitation man, Oklahoma, a post he has held since 1964. Under kinematics at McGill University in 1980. his 22 years of leadership, the Norman laboratory, a President of the Greater Boston chapter of the AMS major research facility of the National Oceanic and At- from 1960 to 1961, Kessler has served on several AMS mospheric Administration (NOAA), has developed the committees including the committees on radar mete- use of Doppler radar in early detection of tornadic de- orology, severe local storms, atmospheric electricity, velopment within thunderstorms. This achievement has and hurricanes and tropical meteorology. He is a fellow permitted as much as 20 minutes of advance warning of the Society and has been a Councilor of the AMS. before a tornado touchdown. Kessler holds an A.B. from Columbia College and a Kessler came to NOAA from the Travelers Research S.M. and Sc.D. from MIT. He was certified as a con- Center in Hartford, Connecticut, where he was senior sulting meteorologist in 1961 and was elected a fellow research scientist from 1961 to 1962 and director of the of the American Association for the Advancement of Atmospheric Physics Division from 1962 to 1964. He Science. Kessler has authored or coauthored approxi- helped to develop applications of radar to meteorology mately 130 reports and publications, principally on ra- in his work in the Cloud Physics Branch and Weather dar meteorology, distribution of water substance in the Radar Branch of the Air Force Cambridge Research Lab- atmosphere (AMS Meteorol. -

Kyle Smith Takes the Helm in Levien Gym
5 MINUTES WITH ... JOHN W. KLUGE ’37, dr. JOHN CLARKE ’93 HISTORY PROFESSOR BUSINESSMAN AND RAPS FOR THE MARTHA HOWELL BENEFACTOR, DIES AT 95 HEALTH OF IT paGE 12 paGE 4 paGE 22 Columbia College TODAY November/December 2010 Kyle Smith Takes the Helm in Levien Gym New men’s basketball coach hopes to lift Lions to next level Thisiv eholiday Your season,sel treatf a yourselfGift. to the benefi ts and privileges of the GColumbia University Club of New York. See how the club and its activities could fit into your life. For more information or to apply, visit www.columbiaclub.org or call (212) 719-0380. The Columbia University Club of New York in residence at 15 West 43 St., New York, NY 10036 Columbia’s SocialIntellectualCulturalRecreationalProfessional Resource in Midtown. Columbia College Today Contents 8 18 14 22 33 24 COVER STORY ALUMNI NEWS DEPARTMENTS 32 2 K YLE SMITH TA K E S THE REIN S B OO ks HELF LETTE rs TO THE 18 Featured: Danielle Evans EDITO R New men’s basketball coach Kyle Smith says if ’04’s debut work, Before You 3 WITHIN THE FAMILY Cornell can climb to the top of the Ivy League, why Suffocate Your Own Fool Self, not Columbia? a collection of short stories. 4 AR OUND THE QUAD S 4 By Alex Sachare ’71 34 Remembering O BITUA R IE S John W. Kluge ’37 6 36 C LA ss NOTE S Austin E. Quigley Theatre Dedicated FEATURES A LUMNI UPDATE S 6 Alumni in the News 54 Dr. -

Getting to Know the WORLD METEOROLOGICAL ORGANIZATION (WMO)
-------~~-~~ \--~-----.------, Qetiuu; /,o Kl«JW tits WoRLD MnEOROLOGICAL ORGANIZATION -• by MILES HARRIS illustrated by VIC DOWD i',. ~__) .( , ~ l V L ) HAQ. G- About the Book In this book we discover that the science of meteorology, as practiced on a world wide scale under the auspices of the United Nations, is far more than a matter of weather prediction - it is a matter of life and death. Following WMO weathermen to the craters of live volcanoes, to locust-plagued Africa and the Middle East, to the war-~orn Congo, we learn two important things about their work. First, the meteorologist is not only a scientist, he is also a kind of missionary, for his job often is to save men from disasters - both the disasters of nature and those which they bring upon themselves. He shows men how to use natural resources to help them live healthier, happier lives. Second, we find that the weatherman may use many sciences in his effort to help and protect us. He might be an expert on radar, earthquakes, or even the habits of insects. Finally, we join these WMO pioneers in their hope to establish a World Weather Watch, which will permit them to bring to all countries the benefits of the scientific understanding which WMO is in a unique position to develop and distribute. About the Illustrator VICTOR DOWD, who shares with his four children the hobby of model-railroading, is an advertising artist as well as an illustrator. He received his art training at Pratt Institute and now lives in Westport, Connecticut. -

Owen-Rhea-Meteorological-Library
May 2014 Owen Rhea’s Meteorological Library 1 John Owen Rhea’s Meteorological Library Inventory Contact Rosemary Rhea: 530-823-0549 [email protected] Topic Directory: General Meteorology 2 Climatology 5 Forecasting and Analysis 9 General Circulation and Fluid Dynamics 12 Tropical Meteorology 17 Air Pollution and Dispersion 19 Mesoscale Modeling 20 Cloud Physics and Convection Modeling 24 Remote Sensing, Instrumentation and Measurement 28 Mountain Meteorology 33 Snow & Quantitative Precipitation Forecasting 35 Hydrology and Snow Hydrology 37 Water Management 41 Weather Modification 44 Colorado Weather Modification 48 Utah Weather Modification 50 California Weather Modification 50 AMS Journals 64 Papers written by Owen Rhea 65 May 2014 Owen Rhea’s Meteorological Library 2 General Meteorology THE ATMOSPHERE - A CHALLENGE The Science of Jute Gregory Charney Richard S. Lindzen, Edward N. Lorenz, George W. Platzman American Meteorological Society Boston, Massachusetts Copyright 1990 by the American Meteorological Society ISBN l-R7R220-03-9 Library of Congress catalog card number 90-81190 PROCEEDINGS OF THE FOREST-ATMOSPHERE INTERACTION WORKSHOP Lake Placid, New York October 1-4, 1985 Published: May 1987 Coordinated and Edited by Harry Moses, Volker A. Mohnen, William E. Reifsnyder, and David H. Slade Workshop cosponsored by: U.S. Department of Energy State University of New York - Albany Yale University UNITED STATES DEPARTMENT OF ENERGY Office of Energy Research Office of Health and Environmental Research Washington, D.C. 20545, New York, Toronto, London Historical Essays on Meteorology 1919-1995 The Diamond Anniversary History Volume of the American Meteorological Society Edited by James Rodger Fleming American Meteorological Society 1996 ISBN 1-878220-17-9 Paper) May 2014 Owen Rhea’s Meteorological Library 3 WEATHER AND LIFE An Introduction to Biometeorology William P. -

Radar in Meteorology Radar in Meteorology: Battan Memorial And
RADAR IN METEOROLOGY RADAR IN METEOROLOGY: BATTAN MEMORIAL AND 40TH ANNIVERSARY RADAR METEOROLOGY CONFERENCE EDITED BY DAVID ATLAS American Meteorological Society Boston 1990 © American Meteorological Society 1990 Originally published by American Meteorological Society Boston in 1990 Softcover reprint of the hardcover 1st edition 1990 Permission to use figures, tables, and brief excerpts from this publication in scientific and educational works is hereby granted, provided the source is acknowledged. All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording, or otherwise, without the prior written permission of the publisher. ISBN 978-0-933876-86-6 ISBN 978-1-935704-15-7 (eBook) DOI 10.1007/978-1-935704-15-7 Typeset and printed in the United States of America by Lancaster Press, Lancaster, Pennsylvania. Section openers designed by Helga Hardy. Published by the American Meteorological Society, 45 Beacon Street, Boston, MA 02176. Richard E. Hallgren, Executive Director Kenneth C. Spengler, Executive Director Emeritus Evelyn Mazur, Assistant Executive Director Arlyn S. Powell, Jr., Publications Manager Editorial support provided by Laura Westlund, Pamela Jones, Jon Feld, Linda Esche, Brenda Gray, Harold Nagel, and Susan McClung. Table of Contents Preface . 1x Acknowledgments . x1 Tribute to Professor Louis J. Battan Xlll I. HISTORY 1 Early Developments of Weather Radar during World War II . 3 ].0. Fletcher 2 Weather Radar in the United States Army's Fort Monmouth Laboratories . 7 Donald M. Swingle 3 Radar Meteorology at Radiation Laboratory, MIT, 1941 to 1947 . 16 Isadore Katz and Patrick f. -

Edwin Kessler National Severe Storms Laboratory National Oceanic and Atmospheric Administration Norman, Okla
Edwin Kessler National Severe Storms Laboratory National Oceanic and Atmospheric Administration Norman, Okla. Abstract sure within and carried off by the wind as their con- The prominent characteristics of tornadoes, their socio- nections are weakened. Only buildings of reinforced logical and meteorological importance, aspects of the concrete and rigidly connected structural steel character- national weather service that pertain to storm forecast- istically escape serious structural damage from violent ing and warning, observational and theoretical studies tornadoes, and windows, roofs, and sidings are always of tornadoes, and some prospects for modifying tor- vulnerable (Brooks, 1951; Flora, 1954; Melaragno, 1968; nadoes, are briefly surveyed. Some paths for future de- Somes et al., 1970). velopment of the warning service and of scientific in- During the last 15 years, about 125 persons have been vestigations are indicated. killed by tornadoes annually, and the annual property damage has averaged about $75 million. These figures 1. General description and significance may be compared with estimated losses caused by light- Tornadoes are among the smallest in horizontal extent ning, hail, and hurricanes as shown in Table 1. of the atmosphere's whirling winds, but they are the Although tornadoes hardly rank as a prominent cause most locally destructive. Although occasionally reported of death in the United States, the number of tornado from Australia, western Europe, India and Japan, it is deaths is highest in relation to property damage -

Weather Modification Today
CONFERENCE PROCEEDINGS ABSTRACTS AND BIOGRAPHICAL SKETCHES LP-2 AUSTIN HILTON INN AUSTIN, TEXAS NOVEMBER 8, 1977 Sponsored by the Texas Water Conservation Association in cooperation with Texas A&M University and the Texas Department of Water Resources Published and distributed by the TEXAS DEPARTMENT OF WATER RESOURCES P.O. Box 13087 Austin, Texas 78711 CONFERENCE PROCEEDINGS ABSTRACTS & BIOGRAPHICAL SKETCHES FOR WEATHER MODIFICATION TODAY AN UPDATE ON THE TECHNOLOGY OPERATIONS RESEARCH SOCIOECONOMIC & LEGAL ASPECTS OF WEATHER MODIFICATION November 8, 1977 Sponsored by the Texas Water Conservation Association in cooperation with Texas A&M University and the Texas Department of Water Resources Austin Hilton Inn IH-35 & HWY 290 Austin, Texas CONTENTS Page AGENDA 3 FOREWORD 4 I. COMMENCEMENT Carl Riehn, President, Texas Water Conservation Association 6 Neville Clarke, ActingDirector, Texas Agricultural Experiment Station, Texas A&M University 7 A. L. Black, Chairman, Texas Water Development Board, Texas Department of Water Resources 8 II. MORNING SESSION Conference Overview, Pierre St. Amand, Naval Weapons Center 10 Texas Programs &Their Objectives, John Carr, Department of Water Resources 12 Project HIPLEX, Lloyd Stuebinger, Bureau of Reclamation 14 Weather Modification Research, T. B. Smith, Meteorology Research, Incorporated 16 Social/Economic Aspects of Weather Modification, Joseph Sonnenfeld/Ronald Lacewell, Texas A&M University 18 Federal Programs and Policy, Merlin Williams, National Oceanic andAtmospheric Administration 22 III. AFTERNOON SESSION Past Storms Related to Agricultural Practices, Edwin Kessler, National Severe Storms Laboratory 26 Legal Implications of WeatherModification, HowardTaubenfeld, Southern Methodist University 28 Texas Projects Colorado River Municipal WaterDistrict,John Girdzus 30 Texas A&M University/HIPLEX, James Scoggins 32 Texas Tech University/HIPLEX, Donald Haragan 34 Meteorology Research, Inc./HIPLEX, T. -

PATH to NEXRAD Doppler Radar Development at the National Severe Storms Laboratory
PATH TO NEXRAD Doppler Radar Development at the National Severe Storms Laboratory BY RODGER A. BROWN AND JOHN M. LEWIS Evolution of events at the National Severe Storms Laboratory during the 1960s and 1970s played an important role in the U.S. government's decision to build the current national network of NEXRAD, or WSR-88D radars. n 1959, 31 people lost their lives in an airplane in developing important new guidelines for the safety accident over Maryland. The fuel tanks in the of flight in the vicinity of thunderstorms (e.g., Kessler IViscount aircraft were struck by lightning and 1990). the resulting explosion and fire led to the fatal crash. In 1962, NSSP placed a research Weather Sur- This event, and several other aircraft accidents that veillance Radar-1957 (WSR-57) radar in Norman, were associated with severe weather, led to the cre- Oklahoma, at the new field facility called the Weather ation of the National Severe Storms Project (NSSP) Radar Laboratory. The following year a decision in 1961 with Clayton Van Thullenar as director and was made to transfer the entire NSSP operation to Chester Newton as chief scientist (Galway 1992). As Norman, where it was reorganized as the National part of NSSP, which was headquartered in Kansas Severe Storms Laboratory (NSSL). Not only was the City, Missouri, Project Rough Rider was established operation of NSSP transferred, but a larger mission to fly instrumented aircraft into storms and compare was envisioned by the new U.S. Weather Bureau the relationship of radar echo strength with aircraft- (USWB) chief, Robert White. -
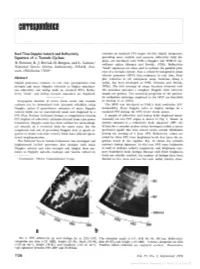
Correspondence
correspondence Real Time Doppler Isotach and Reflectivity contours on standard PPI scopes. On-line digital integrators, Signature of a Tornado Cyclone providing more reliable and accurate reflectivity field dis- 1 plays, are interfaced with NSSL's Doppler and WSR-57 sur- D. Sirmans, R. J. Doviak, D. Burgess, and L. Lemon, veillance radars (Sirmans and Doviak, 1973b). Reflectivity National Severe Storms Laboratory, NOAA, Nor- "hook" signatures are often used to indicate the possible pres- man, Oklahoma 73069 ence of a tornado cyclone. Now a relatively inexpensive mean velocity processor (MVP) that estimates, in real time, Dop- Abstract pler velocities at all contiguous range locations along a Digital processors estimate, in real time, precipitation echo radial, has been developed at NSSL (Sirmans and Doviak, strength and mean Doppler velocities to display simultane- 1973a). The full coverage of range locations obtained with ous reflectivity and isodop fields on standard PPI's. Reflec- this processor provides a complete Doppler field wherever tivity "hook" and isodop tornado signatures are displayed. targets are present. The statistical properties of the particu- lar estimation technique employed in the MVP are described Geographic location of severe storm winds and tornado by Doviak et al (1974). cyclones can be determined with increased reliability using The MVP was interfaced to NSSL's high resolution (0.8° Doppler radars if quantitative estimates of mean Doppler beamwidth), 10-cm Doppler radar to display isodops on a velocity fields can be conveniently made and displayed in a standard PPI during the 1973 severe storm season. PPI (Plan Position Indicator) format to complement existing A sample of reflectivity and isodop fields displayed simul- PPI displays of reflectivity estimates derived from echo power taneously on two PPI scopes is shown in Fig. -

JC Library University of Oklahoma
---.. -------)- U.S. DEPARTMENT OF COMMERCE ENVIRONMENTAL SCIENCE SERVICES ADMINISTRATIQN INSTITUTES FOR ENVIRONMENTAL RESEARCH Institutes for Environmental Research Technical Memorandum -NSSL 34 NOTE ON PROBING BALLOON MOTION BY DOPPLER RADAR Roger M. Lhermitte property of NV>;JC Library University of Oklahoma NATIONAL SEVERE STORMS LABORATORY TECHNICAL MEMORANDUM NO. 34 NORMAN, OKLAHOMA JULY 1967 TABLE OF CONTENTS Page Abstract 1 . 1 Introduction and Method 1 2 The Balloon Sel£-Induced Turbulence 2 3 Balloon Motion Variability and Its Relationship to 4 Balloon Size and Ascent Rate ,. 4 Conclusion 13 Acknowledgments 13 Re£erences 14 iii· LIST OF FIGURES Page 1 Effect on balloon motion caused by balloon burst 3 2 Time variations of velocity compared to the motion 5 of surrounding ice cryst~ls 3 Time variations of the radial speed of small 5 balloon (D = 12") 4 Helicoidal trajectory parameters~ associated with 6 the sinusoidal variation of balloon speed 5 Change of the 100-gm balloon motion with the 8 vertical lift or Reynolds number 6 Time variations of Doppler speed for 30-gm~ 24 inch 9 diameters with different vertical ascent rate 7 Relationship between A and Rd for 42 flights 10 8 Relationship between A and balloon diameter D 10 9 Relationship between A and w (24 flights) 10 10 Relationship between orbit radius and balloon 11 diameter for 26 flights 11 Relationship of orbit radius~ r~ to balloon 11 diameter~ D~ as a function of Reynolds number Rd 12 Relationship between riD and the balloon relative 12 mass as proposed by MacCready (15 flights) iv NOTE ON PROBING BALLOON MOTION BY DOPPLER RADARl ,2 RogerM. -

165 Program of the Tenth Weather Radar Conference, April 22-25, 1963, Washington, D. C
VOL. 44, No. 3, MARCH 1963 165 Program of the Tenth Weather Radar Conference, April 22-25, 1963, Washington, D. C. National Officers District of Columbia Branch Officers President: Prof. Morris Neiburger F. W. Burnett, Chairman Vice President: Dr. Helmut E. Landsberg David Johnson, Vice Chairman Secretary: Prof. James E. Miller John Webber, Secretary Treasurer: Henry DeC. Ward James Neilon, Treasurer Executive Secretary: Kenneth C. Spengler Morris Tepper, Member-at-Large Committee on Radar Meteorology Conference Committee Pauline M. Austin, Chairman Stuart G. Bigler, Chairman Stuart G. Bigler Vaughn D. Rockney Harrie E. Foster, Jr. Allen F. Flanders Homer W. Hiser Robert W. Decker Walter Hitschfeld Fred E. Wells Edwin Kessler, III Paul L. Hexter, Jr. GENERAL INFORMATION PROGRAM The Tenth Weather Radar Conference will be held at Monday Morning, 22 April, 9-12 a.m. the International Inn, Thomas Circle. The meeting will be under the joint sponsorship of the U. S. Weather USE OF RADAR IN FORECASTING Bureau and the American Meteorological Society and Chairman: Dr. Edwin Kessler, III will be hosted by the District of Columbia Branch of the The Travelers Research Center Society. Hartford, Conn. Rooms have been reserved at the International Inn for Radar precipitation echo motion and suggested prediction conferees and their guests. Single room rates range from techniques. Roland J. Boucher, Aracon Laboratories. $12 to $18 per day, double rooms from $15 to $24 per day. Reservations should be made early since a number of (15 min) other conferences and conventions and a large number of Some studies on the shape and movement of a weather tourists should be in town at that time.