Transcriptional Regulation After Chronic Hypoxia Exposure In
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Supplemental Information to Mammadova-Bach Et Al., “Laminin Α1 Orchestrates VEGFA Functions in the Ecosystem of Colorectal Carcinogenesis”
Supplemental information to Mammadova-Bach et al., “Laminin α1 orchestrates VEGFA functions in the ecosystem of colorectal carcinogenesis” Supplemental material and methods Cloning of the villin-LMα1 vector The plasmid pBS-villin-promoter containing the 3.5 Kb of the murine villin promoter, the first non coding exon, 5.5 kb of the first intron and 15 nucleotides of the second villin exon, was generated by S. Robine (Institut Curie, Paris, France). The EcoRI site in the multi cloning site was destroyed by fill in ligation with T4 polymerase according to the manufacturer`s instructions (New England Biolabs, Ozyme, Saint Quentin en Yvelines, France). Site directed mutagenesis (GeneEditor in vitro Site-Directed Mutagenesis system, Promega, Charbonnières-les-Bains, France) was then used to introduce a BsiWI site before the start codon of the villin coding sequence using the 5’ phosphorylated primer: 5’CCTTCTCCTCTAGGCTCGCGTACGATGACGTCGGACTTGCGG3’. A double strand annealed oligonucleotide, 5’GGCCGGACGCGTGAATTCGTCGACGC3’ and 5’GGCCGCGTCGACGAATTCACGC GTCC3’ containing restriction site for MluI, EcoRI and SalI were inserted in the NotI site (present in the multi cloning site), generating the plasmid pBS-villin-promoter-MES. The SV40 polyA region of the pEGFP plasmid (Clontech, Ozyme, Saint Quentin Yvelines, France) was amplified by PCR using primers 5’GGCGCCTCTAGATCATAATCAGCCATA3’ and 5’GGCGCCCTTAAGATACATTGATGAGTT3’ before subcloning into the pGEMTeasy vector (Promega, Charbonnières-les-Bains, France). After EcoRI digestion, the SV40 polyA fragment was purified with the NucleoSpin Extract II kit (Machery-Nagel, Hoerdt, France) and then subcloned into the EcoRI site of the plasmid pBS-villin-promoter-MES. Site directed mutagenesis was used to introduce a BsiWI site (5’ phosphorylated AGCGCAGGGAGCGGCGGCCGTACGATGCGCGGCAGCGGCACG3’) before the initiation codon and a MluI site (5’ phosphorylated 1 CCCGGGCCTGAGCCCTAAACGCGTGCCAGCCTCTGCCCTTGG3’) after the stop codon in the full length cDNA coding for the mouse LMα1 in the pCIS vector (kindly provided by P. -

Effect of Citric Acid Cycle Genetic Variants and Their Interactions With
cancers Article Effect of Citric Acid Cycle Genetic Variants and Their Interactions with Obesity, Physical Activity and Energy Intake on the Risk of Colorectal Cancer: Results from a Nested Case-Control Study in the UK Biobank Sooyoung Cho 1 , Nan Song 2,3 , Ji-Yeob Choi 2,4,5 and Aesun Shin 1,2,* 1 Department of Preventive Medicine, Seoul National University College of Medicine, Seoul 03080, Korea; [email protected] 2 Cancer Research Institute, Seoul National University, Seoul 03080, Korea; [email protected] (N.S.); [email protected] (J.-Y.C.) 3 Department of Epidemiology and Cancer Control, St. Jude Children’s Research Hospital, Memphis, TN 38105, USA 4 Department of Biomedical Sciences, Graduate School of Seoul National University, Seoul 03080, Korea 5 Medical Research Center, Institute of Health Policy and Management, Seoul National University, Seoul 03080, Korea * Correspondence: [email protected]; Tel.: +82-2-740-8331; Fax: +82-2-747-4830 Received: 18 August 2020; Accepted: 9 October 2020; Published: 12 October 2020 Simple Summary: The citric acid cycle has a central role in the cellular energy metabolism and biosynthesis of macromolecules in the mitochondrial matrix. We identified the single nucleotide polymorphisms (SNPs) of the citrate acid cycle with colorectal cancer susceptibility in UK population. Furthermore, we found the significant interaction of SNPs in the citric acid cycle with the contributors to energy balance and SNP-SNP interactions. Our findings provide clues to the etiology in cancer development related to energy metabolism and evidence on identification of the population at high risk of colorectal cancer. -

Molecular Profile of Tumor-Specific CD8+ T Cell Hypofunction in a Transplantable Murine Cancer Model
Downloaded from http://www.jimmunol.org/ by guest on September 25, 2021 T + is online at: average * The Journal of Immunology , 34 of which you can access for free at: 2016; 197:1477-1488; Prepublished online 1 July from submission to initial decision 4 weeks from acceptance to publication 2016; doi: 10.4049/jimmunol.1600589 http://www.jimmunol.org/content/197/4/1477 Molecular Profile of Tumor-Specific CD8 Cell Hypofunction in a Transplantable Murine Cancer Model Katherine A. Waugh, Sonia M. Leach, Brandon L. Moore, Tullia C. Bruno, Jonathan D. Buhrman and Jill E. Slansky J Immunol cites 95 articles Submit online. Every submission reviewed by practicing scientists ? is published twice each month by Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts http://jimmunol.org/subscription Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html http://www.jimmunol.org/content/suppl/2016/07/01/jimmunol.160058 9.DCSupplemental This article http://www.jimmunol.org/content/197/4/1477.full#ref-list-1 Information about subscribing to The JI No Triage! Fast Publication! Rapid Reviews! 30 days* Why • • • Material References Permissions Email Alerts Subscription Supplementary The Journal of Immunology The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2016 by The American Association of Immunologists, Inc. All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. This information is current as of September 25, 2021. The Journal of Immunology Molecular Profile of Tumor-Specific CD8+ T Cell Hypofunction in a Transplantable Murine Cancer Model Katherine A. -

Chromosomal Aberrations in Head and Neck Squamous Cell Carcinomas in Norwegian and Sudanese Populations by Array Comparative Genomic Hybridization
825-843 12/9/08 15:31 Page 825 ONCOLOGY REPORTS 20: 825-843, 2008 825 Chromosomal aberrations in head and neck squamous cell carcinomas in Norwegian and Sudanese populations by array comparative genomic hybridization ERIC ROMAN1,2, LEONARDO A. MEZA-ZEPEDA3, STINE H. KRESSE3, OLA MYKLEBOST3,4, ENDRE N. VASSTRAND2 and SALAH O. IBRAHIM1,2 1Department of Biomedicine, Faculty of Medicine and Dentistry, University of Bergen, Jonas Lies vei 91; 2Department of Oral Sciences - Periodontology, Faculty of Medicine and Dentistry, University of Bergen, Årstadveien 17, 5009 Bergen; 3Department of Tumor Biology, Institute for Cancer Research, Rikshospitalet-Radiumhospitalet Medical Center, Montebello, 0310 Oslo; 4Department of Molecular Biosciences, University of Oslo, Blindernveien 31, 0371 Oslo, Norway Received January 30, 2008; Accepted April 29, 2008 DOI: 10.3892/or_00000080 Abstract. We used microarray-based comparative genomic logical parameters showed little correlation, suggesting an hybridization to explore genome-wide profiles of chromosomal occurrence of gains/losses regardless of ethnic differences and aberrations in 26 samples of head and neck cancers compared clinicopathological status between the patients from the two to their pair-wise normal controls. The samples were obtained countries. Our findings indicate the existence of common from Sudanese (n=11) and Norwegian (n=15) patients. The gene-specific amplifications/deletions in these tumors, findings were correlated with clinicopathological variables. regardless of the source of the samples or attributed We identified the amplification of 41 common chromosomal carcinogenic risk factors. regions (harboring 149 candidate genes) and the deletion of 22 (28 candidate genes). Predominant chromosomal alterations Introduction that were observed included high-level amplification at 1q21 (harboring the S100A gene family) and 11q22 (including Head and neck squamous cell carcinoma (HNSCC), including several MMP family members). -

Defining Functional Interactions During Biogenesis of Epithelial Junctions
ARTICLE Received 11 Dec 2015 | Accepted 13 Oct 2016 | Published 6 Dec 2016 | Updated 5 Jan 2017 DOI: 10.1038/ncomms13542 OPEN Defining functional interactions during biogenesis of epithelial junctions J.C. Erasmus1,*, S. Bruche1,*,w, L. Pizarro1,2,*, N. Maimari1,3,*, T. Poggioli1,w, C. Tomlinson4,J.Lees5, I. Zalivina1,w, A. Wheeler1,w, A. Alberts6, A. Russo2 & V.M.M. Braga1 In spite of extensive recent progress, a comprehensive understanding of how actin cytoskeleton remodelling supports stable junctions remains to be established. Here we design a platform that integrates actin functions with optimized phenotypic clustering and identify new cytoskeletal proteins, their functional hierarchy and pathways that modulate E-cadherin adhesion. Depletion of EEF1A, an actin bundling protein, increases E-cadherin levels at junctions without a corresponding reinforcement of cell–cell contacts. This unexpected result reflects a more dynamic and mobile junctional actin in EEF1A-depleted cells. A partner for EEF1A in cadherin contact maintenance is the formin DIAPH2, which interacts with EEF1A. In contrast, depletion of either the endocytic regulator TRIP10 or the Rho GTPase activator VAV2 reduces E-cadherin levels at junctions. TRIP10 binds to and requires VAV2 function for its junctional localization. Overall, we present new conceptual insights on junction stabilization, which integrate known and novel pathways with impact for epithelial morphogenesis, homeostasis and diseases. 1 National Heart and Lung Institute, Faculty of Medicine, Imperial College London, London SW7 2AZ, UK. 2 Computing Department, Imperial College London, London SW7 2AZ, UK. 3 Bioengineering Department, Faculty of Engineering, Imperial College London, London SW7 2AZ, UK. 4 Department of Surgery & Cancer, Faculty of Medicine, Imperial College London, London SW7 2AZ, UK. -

Mtor Coordinates Transcriptional Programs and Mitochondrial Metabolism of Activated Treg Subsets to Protect Tissue Homeostasis
ARTICLE DOI: 10.1038/s41467-018-04392-5 OPEN mTOR coordinates transcriptional programs and mitochondrial metabolism of activated Treg subsets to protect tissue homeostasis Nicole M. Chapman1, Hu Zeng 1, Thanh-Long M. Nguyen1, Yanyan Wang1, Peter Vogel 2, Yogesh Dhungana1, Xiaojing Liu3, Geoffrey Neale 4, Jason W. Locasale 3 & Hongbo Chi1 1234567890():,; Regulatory T (Treg) cells derived from the thymus (tTreg) and periphery (pTreg) have central and distinct functions in immunosuppression, but mechanisms for the generation and acti- vation of Treg subsets in vivo are unclear. Here, we show that mechanistic target of rapamycin (mTOR) unexpectedly supports the homeostasis and functional activation of tTreg and pTreg cells. mTOR signaling is crucial for programming activated Treg-cell function to protect immune tolerance and tissue homeostasis. Treg-specific deletion of mTOR drives sponta- neous effector T-cell activation and inflammation in barrier tissues and is associated with reduction in both thymic-derived effector Treg (eTreg) and pTreg cells. Mechanistically, mTOR functions downstream of antigenic signals to drive IRF4 expression and mitochondrial metabolism, and accordingly, deletion of mitochondrial transcription factor A (Tfam) severely impairs Treg-cell suppressive function and eTreg-cell generation. Collectively, our results show that mTOR coordinates transcriptional and metabolic programs in activated Treg subsets to mediate tissue homeostasis. 1 Department of Immunology, St. Jude Children’s Research Hospital, 262 Danny Thomas Place, MS 351, Memphis, TN 38105, USA. 2 Department of Pathology, St. Jude Children’s Research Hospital, 262 Danny Thomas Place, MS 250, Memphis, TN 38105, USA. 3 Department of Pharmacology & Cancer Biology, Duke University School of Medicine, Levine Science Research Center C266, Box 3813, Durham, NC 27710, USA. -

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -

Communication Pathways in Human Nonmuscle Myosin-2C 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 Authors: 25 Krishna Chinthalapudia,B,C,1, Sarah M
1 Mechanistic Insights into the Active Site and Allosteric 2 Communication Pathways in Human Nonmuscle Myosin-2C 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 Authors: 25 Krishna Chinthalapudia,b,c,1, Sarah M. Heisslera,d,1, Matthias Prellera,e, James R. Sellersd,2, and 26 Dietmar J. Mansteina,b,2 27 28 Author Affiliations 29 aInstitute for Biophysical Chemistry, OE4350 Hannover Medical School, 30625 Hannover, 30 Germany. 31 bDivision for Structural Biochemistry, OE8830, Hannover Medical School, 30625 Hannover, 32 Germany. 33 cCell Adhesion Laboratory, Department of Integrative Structural and Computational Biology, The 34 Scripps Research Institute, Jupiter, Florida 33458, USA. 35 dLaboratory of Molecular Physiology, NHLBI, National Institutes of Health, Bethesda, Maryland 36 20892, USA. 37 eCentre for Structural Systems Biology (CSSB), German Electron Synchrotron (DESY), 22607 38 Hamburg, Germany. 39 1K.C. and S.M.H. contributed equally to this work 40 2To whom correspondence may be addressed: E-mail: [email protected] or 41 [email protected] 42 43 1 44 Abstract 45 Despite a generic, highly conserved motor domain, ATP turnover kinetics and their activation by 46 F-actin vary greatly between myosin-2 isoforms. Here, we present a 2.25 Å crystal pre- 47 powerstroke state (ADPVO4) structure of the human nonmuscle myosin-2C motor domain, one 48 of the slowest myosins characterized. In combination with integrated mutagenesis, ensemble- 49 solution kinetics, and molecular dynamics simulation approaches, the structure reveals an 50 allosteric communication pathway that connects the distal end of the motor domain with the 51 active site. -
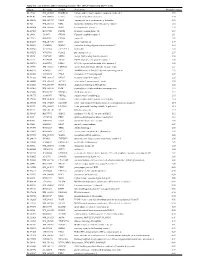
Supplementary Table 1
Table S1. List of Genes Differentially Expressed in YB-1 siRNA-Transfected MCF-7 Cells Unigene Accession Symbol Description Mean fold change Hs.17466 NM_004585 RARRES3 retinoic acid receptor responder (tazarotene induced) 3 3.57 Hs.64746 NM_004669 CLIC3 chloride intracellular channel 3 3.33 Hs.516155 NM_001747 CAPG capping protein (actin filament), gelsolin-like 2.88 Hs.926 NM_002463 MX2 myxovirus (influenza virus) resistance 2 (mouse) 2.72 Hs.643494 NM_005410 SEPP1 selenoprotein P, plasma, 1 2.70 Hs.267038 BC017500 POF1B premature ovarian failure 1B 2.67 Hs.20961 U63917 GPR30 G protein- coupled receptor 30 2532.53 Hs.199877 BE645967 CPNE4 copine IV 2.49 Hs.632177 NM_017458 MVP major vault protein 2.48 Hs.528836 AA005023 NOD27 nucleotide-binding oligomerization domains 27 2.43 Hs.360940 AL589866 dJ222E13.1 kraken-like 2.40 Hs.515575 AI743780 PLAC2 placenta-specific 2 2.37 Hs.25674 AI827820 MBD2 methyl-CpG binding domain protein 2 2.36 Hs.12229 AA149594 TIEG2 TGFB inducible early growth response 2 2.33 Hs.482730 AA053711 EDIL3 EGF-like repeats and discoidin I-like domains 3 2.32 Hs. 370771 NM_000389 CDKN1A cyclin- depen dent kinase in hibitor 1A (p 21, Cip 1) 2282.28 Hs.469175 AI341537 JFC1 NADPH oxidase-related, C2 domain-containing protein 2.28 Hs.514821 AF043341 CCL5 chemokine (C-C motif) ligand 5 2.27 Hs.591292 NM_023915 GPR87 G protein-coupled receptor 87 2.26 Hs.500483 NM_001613 ACTA2 actin, alpha 2, smooth muscle, aorta 2.24 Hs.632824 NM_006729 DIAPH2 diaphanous homolog 2 (Drosophila) 2.22 Hs.369430 NM_000919 PAM peptidylglycine -

Anti-IDH3A Antibody (ARG42205)
Product datasheet [email protected] ARG42205 Package: 100 μl anti-IDH3A antibody Store at: -20°C Summary Product Description Rabbit Polyclonal antibody recognizes IDH3A Tested Reactivity Hu, Ms, Rat Tested Application ICC/IF, IHC-P, WB Host Rabbit Clonality Polyclonal Isotype IgG Target Name IDH3A Antigen Species Human Immunogen Recombinant fusion protein corresponding to aa. 28-366 of Human IDH3A (NP_005521.1). Conjugation Un-conjugated Alternate Names Isocitrate dehydrogenase [NAD] subunit alpha, mitochondrial; EC 1.1.1.41; NAD; Isocitric dehydrogenase subunit alpha; + Application Instructions Application table Application Dilution ICC/IF 1:50 - 1:200 IHC-P 1:50 - 1:200 WB 1:500 - 1:2000 Application Note * The dilutions indicate recommended starting dilutions and the optimal dilutions or concentrations should be determined by the scientist. Positive Control Raji Calculated Mw 40 kDa Observed Size ~ 40 kDa Properties Form Liquid Purification Affinity purified. Buffer PBS (pH 7.3), 0.02% Sodium azide and 50% Glycerol. Preservative 0.02% Sodium azide Stabilizer 50% Glycerol Storage instruction For continuous use, store undiluted antibody at 2-8°C for up to a week. For long-term storage, aliquot and store at -20°C. Storage in frost free freezers is not recommended. Avoid repeated freeze/thaw www.arigobio.com 1/3 cycles. Suggest spin the vial prior to opening. The antibody solution should be gently mixed before use. Note For laboratory research only, not for drug, diagnostic or other use. Bioinformation Gene Symbol IDH3A Gene Full Name isocitrate dehydrogenase 3 (NAD+) alpha Background Isocitrate dehydrogenases catalyze the oxidative decarboxylation of isocitrate to 2-oxoglutarate. -

NICU Gene List Generator.Xlsx
Neonatal Crisis Sequencing Panel Gene List Genes: A2ML1 - B3GLCT A2ML1 ADAMTS9 ALG1 ARHGEF15 AAAS ADAMTSL2 ALG11 ARHGEF9 AARS1 ADAR ALG12 ARID1A AARS2 ADARB1 ALG13 ARID1B ABAT ADCY6 ALG14 ARID2 ABCA12 ADD3 ALG2 ARL13B ABCA3 ADGRG1 ALG3 ARL6 ABCA4 ADGRV1 ALG6 ARMC9 ABCB11 ADK ALG8 ARPC1B ABCB4 ADNP ALG9 ARSA ABCC6 ADPRS ALK ARSL ABCC8 ADSL ALMS1 ARX ABCC9 AEBP1 ALOX12B ASAH1 ABCD1 AFF3 ALOXE3 ASCC1 ABCD3 AFF4 ALPK3 ASH1L ABCD4 AFG3L2 ALPL ASL ABHD5 AGA ALS2 ASNS ACAD8 AGK ALX3 ASPA ACAD9 AGL ALX4 ASPM ACADM AGPS AMELX ASS1 ACADS AGRN AMER1 ASXL1 ACADSB AGT AMH ASXL3 ACADVL AGTPBP1 AMHR2 ATAD1 ACAN AGTR1 AMN ATL1 ACAT1 AGXT AMPD2 ATM ACE AHCY AMT ATP1A1 ACO2 AHDC1 ANK1 ATP1A2 ACOX1 AHI1 ANK2 ATP1A3 ACP5 AIFM1 ANKH ATP2A1 ACSF3 AIMP1 ANKLE2 ATP5F1A ACTA1 AIMP2 ANKRD11 ATP5F1D ACTA2 AIRE ANKRD26 ATP5F1E ACTB AKAP9 ANTXR2 ATP6V0A2 ACTC1 AKR1D1 AP1S2 ATP6V1B1 ACTG1 AKT2 AP2S1 ATP7A ACTG2 AKT3 AP3B1 ATP8A2 ACTL6B ALAS2 AP3B2 ATP8B1 ACTN1 ALB AP4B1 ATPAF2 ACTN2 ALDH18A1 AP4M1 ATR ACTN4 ALDH1A3 AP4S1 ATRX ACVR1 ALDH3A2 APC AUH ACVRL1 ALDH4A1 APTX AVPR2 ACY1 ALDH5A1 AR B3GALNT2 ADA ALDH6A1 ARFGEF2 B3GALT6 ADAMTS13 ALDH7A1 ARG1 B3GAT3 ADAMTS2 ALDOB ARHGAP31 B3GLCT Updated: 03/15/2021; v.3.6 1 Neonatal Crisis Sequencing Panel Gene List Genes: B4GALT1 - COL11A2 B4GALT1 C1QBP CD3G CHKB B4GALT7 C3 CD40LG CHMP1A B4GAT1 CA2 CD59 CHRNA1 B9D1 CA5A CD70 CHRNB1 B9D2 CACNA1A CD96 CHRND BAAT CACNA1C CDAN1 CHRNE BBIP1 CACNA1D CDC42 CHRNG BBS1 CACNA1E CDH1 CHST14 BBS10 CACNA1F CDH2 CHST3 BBS12 CACNA1G CDK10 CHUK BBS2 CACNA2D2 CDK13 CILK1 BBS4 CACNB2 CDK5RAP2 -

Kras, Braf, Erbb2, Tp53
Supplementary Table 1. SBT-EOC cases used for molecular analyses Fresh Frozen Tissue FFPE Tissue Tumour HRM Case ID PCR-Seq HRM component Oncomap (KRAS, BRAF, CNV GE CNV IHC (NRAS) (TP53 Only) ERBB2, TP53) Paired Cases 15043 SBT & INV √ √ √ √ √ √ 65662 SBT & INV √ √ √ √ √ 65661 SBT & INV √ √ √ √ √ √ 9128 SBT & INV √ √ √ √ √ 2044 SBT & INV √ √ √ √ √ 3960 SBT & INV √ √ √ √ √ 5899 SBT & INV √ √ √ √ √ 65663 SBT & INV √ √ √ Inv √ √ √ 65666 SBT √ Inv √ √ √ √ √ 65664 SBT √ √ √ Inv √ √ √ √ √ 15060 SBT √ √ √ Inv √ √ √ √ √ 65665 SBT √ √ √ Inv √ √ √ √ √ 65668 SBT √ √ √ Inv √ √ √ √ 65667 SBT √ √ √ Inv √ √ √ √ 15018 SBT √ Inv √ √ √ √ 15071 SBT √ Inv √ √ √ √ 65670 SBT √ Inv √ √ √ 8982 SBT √ Unpaired Cases 15046 Inv √ √ √ 15014 Inv √ √ √ 65671 Inv √ √ √ 65672 SBT √ √ √ 65673 Inv √ √ √ 65674 Inv √ √ √ 65675 Inv √ √ √ 65676 Inv √ √ √ 65677 Inv √ √ √ 65678 Inv √ √ √ 65679 Inv √ √ √ Fresh Frozen Tissue FFPE Tissue Tumour HRM Case ID PCR-Seq HRM component Oncomap (KRAS, BRAF, CNV GE CNV IHC (NRAS) (TP53 Only) ERBB2, TP53) 65680 Inv √ √ √ 5349 Inv √ √ √ 7957 Inv √ √ √ 8395 Inv √ √ √ 8390 Inv √ √ 2110 Inv √ √ 6328 Inv √ √ 9125 Inv √ √ 9221 Inv √ √ 10701 Inv √ √ 1072 Inv √ √ 11266 Inv √ √ 11368 Inv √ √ √ 12237 Inv √ √ 2064 Inv √ √ 3539 Inv √ √ 2189 Inv √ √ √ 5711 Inv √ √ 6251 Inv √ √ 6582 Inv √ √ 7200 SBT √ √ √ 8633 Inv √ √ √ 9579 Inv √ √ 10740 Inv √ √ 11766 Inv √ √ 3958 Inv √ √ 4723 Inv √ √ 6244 Inv √ √ √ 7716 Inv √ √ SBT-EOC, serous carcinoma with adjacent borderline regions; SBT, serous borderline component of SBT- EOC; Inv, invasive component of SBT-EOC;