Modulation of Conduction and Refractoriness in Atrioventricular Junctional Reentrant Circuit. Effect on Reentry Initiated by Atrial Extrastimulus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Rhythms & Cardiac Emergencies
Rhythm & 12 Lead EKG Review March 2011 CE Condell Medical Center EMS System Site code # 107200E-1211 Prepared by: FF/PMD Michael Mounts – Lake Forest Fire Revised By: Sharon Hopkins, RN, BSN, EMT-P Objectives Upon successful completion of this module, the EMS provider will be able to: • Identify the components of a rhythm strip • Identify what the components represent on the rhythm strip • Identify criteria for sinus rhythms • Identify criteria for atrial rhythms • Identify AV/junctional rhythms Objectives cont. • Identify ventricular rhythms • Identify rhythms with AV blocks • Identify treatments for different rhythms • Identify criteria for identification of ST elevation on 12 lead EKG’s • Identify EMS treatment for patients with acute coronary syndrome (ST elevation) • Demonstrate standard & alternate placement of ECG electrodes for monitoring Objectives cont. • Demonstrate placement of electrodes for obtaining a 12 lead EKG • Demonstrate the ability to identify a variety of static or dynamic EKG rhythm strips • Demonstrate the ability to identify the presence or absence of ST elevation when presented with a 12 lead EKG • Review department’s process to transmit 12 lead EKG to hospital, if capable • Successfully complete the post quiz with a score of 80% or better. ECG Paper • What do the boxes represent? • How do you measure time & amplitude? Components of the Rhythm Strip • ECG Paper • Wave forms • Wave complexes • Wave segments • Wave intervals Wave Forms, Complexes, Segments & Intervals • P wave – atrial depolarization • QRS – Ventricular -

View Pdf Copy of Original Document
Phenotype definition for the Vanderbilt Genome-Electronic Records project Identifying genetics determinants of normal QRS duration (QRSd) Patient population: • Patients with DNA whose first electrocardiogram (ECG) is designated as “normal” and lacking an exclusion criteria. • For this study, case and control are drawn from the same population and analyzed via continuous trait analysis. The only difference will be the QRSd. Hypothetical timeline for a single patient: Notes: • The study ECG is the first normal ECG. • The “Mildly abnormal” ECG cannot be abnormal by presence of heart disease. It can have abnormal rate, be recorded in the presence of Na-channel blocking meds, etc. For instance, a HR >100 is OK but not a bundle branch block. • Y duration = from first entry in the electronic medical record (EMR) until one month following normal ECG • Z duration = most recent clinic visit or problem list (if present) to one week following the normal ECG. Labs values, though, must be +/- 48h from the ECG time Criteria to be included in the analysis: Criteria Source/Method “Normal” ECG must be: • QRSd between 65-120ms ECG calculations • ECG designed as “NORMAL” ECG classification • Heart Rate between 50-100 ECG calculations • ECG Impression must not contain Natural Language Processing (NLP) on evidence of heart disease concepts (see ECG impression. Will exclude all but list below) negated terms (e.g., exclude those with possible, probable, or asserted bundle branch blocks). Should also exclude normalization negations like “LBBB no longer present.” -
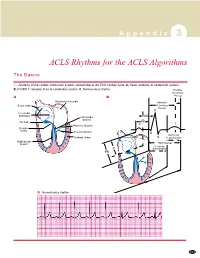
ACLS Rhythms for the ACLS Algorithms
A p p e n d i x 3 ACLS Rhythms for the ACLS Algorithms The Basics 1. Anatomy of the cardiac conduction system: relationship to the ECG cardiac cycle. A, Heart: anatomy of conduction system. B, P-QRS-T complex: lines to conduction system. C, Normal sinus rhythm. Relative Refractory A B Period Bachmann’s bundle Absolute Sinus node Refractory Period R Internodal pathways Left bundle AVN branch AV node PR T Posterior division P Bundle of His Anterior division Q Ventricular Purkinje fibers S Repolarization Right bundle branch QT Interval Ventricular P Depolarization PR C Normal sinus rhythm 253 A p p e n d i x 3 The Cardiac Arrest Rhythms 2. Ventricular Fibrillation/Pulseless Ventricular Tachycardia Pathophysiology ■ Ventricles consist of areas of normal myocardium alternating with areas of ischemic, injured, or infarcted myocardium, leading to chaotic pattern of ventricular depolarization Defining Criteria per ECG ■ Rate/QRS complex: unable to determine; no recognizable P, QRS, or T waves ■ Rhythm: indeterminate; pattern of sharp up (peak) and down (trough) deflections ■ Amplitude: measured from peak-to-trough; often used subjectively to describe VF as fine (peak-to- trough 2 to <5 mm), medium-moderate (5 to <10 mm), coarse (10 to <15 mm), very coarse (>15 mm) Clinical Manifestations ■ Pulse disappears with onset of VF ■ Collapse, unconsciousness ■ Agonal breaths ➔ apnea in <5 min ■ Onset of reversible death Common Etiologies ■ Acute coronary syndromes leading to ischemic areas of myocardium ■ Stable-to-unstable VT, untreated ■ PVCs with -

Dysrhythmias
CARDIOVASCULAR DISORDERS DYSRHYTHMIAS I. BASIC PRINCIPLES OF CARDIAC CONDUCTION DISTURBANCES A. Standard ECG and rhythm strips 1. Recordings are obtained at a paper speed of 25 mm/sec. 2. The vertical axis measures distance; the smallest divisions are 1 mm ×1 mm. 3. The horizontal axis measures time; each small division is 0.04 sec/mm. B. Normal morphology Courtesy of Dr. Michael McCrea 1. P wave = atrial depolarization a. Upright in leads I, II, III, aVL, and aVF; inverted in lead aVR b. Measures <0.10 seconds wide and <3 mm high c. Normal PR interval is 0.12–0.20 seconds. 2. QRS complex = ventricular depolarization a. Measures 0.06-0.10 seconds wide b. Q wave (1) <0.04 seconds wide and <3 mm deep (2) Abnormal if it is >3 mm deep or >1/3 of the QRS complex. c. R wave ≤7.5 mm high 3. QT interval varies with rate and sex but is usually 0.33–0.42 seconds; at normal heart rates, it is normally <1/2 the preceding RR interval. 4. T wave = ventricular repolarization a. Upright in leads I, II, V3–V6; inverted in aVR b. Slightly rounded and asymmetric in configuration c. Measures ≤5 mm high in limb leads and ≤10 mm high in the chest leads 5. U wave = a ventricular afterpotential a. Any deflection after the T wave (usually low voltage) b. Same polarity as the T wave c. Most easily detected in lead V3 d. Can be a normal component of the ECG e. Prominent U waves may indicate one of the following: (1) Hypokalemia (<3 mEq/L) (2) Hypercalcemia (3) Therapy with digitalis, phenothiazines, quinidine, epinephrine, inotropic agents, or amiodarone (4) Thyrotoxicosis f. -

(EHRA) Consensus Document on the Management of Supraventricular Arrhythmias, Endorsed by Heart
Europace Advance Access published November 17, 2016 Europace EHRA POSITION PAPER doi:10.1093/europace/euw301 European Heart Rhythm Association (EHRA) consensus document on the management of supraventricular arrhythmias, endorsed by Heart Rhythm Society (HRS), Asia-Pacific Heart Rhythm Society (APHRS), and Sociedad Latinoamericana de Estimulacio´nCardiacay Electrofisiologia (SOLAECE) Demosthenes G. Katritsis, (Chair)1, Giuseppe Boriani2,FranciscoG.Cosio3, Gerhard Hindricks4,PierreJaı¨s5,MarkE.Josephson6, Roberto Keegan7, Young-Hoon Kim8, Bradley P. Knight9,Karl-HeinzKuck10,DeirdreA.Lane10,11, Downloaded from GregoryY.H.Lip11, Helena Malmborg12, Hakan Oral13, Carlo Pappone14, 15 16 Sakis Themistoclakis ,KathrynA.Wood , and Carina Blomstro¨m-Lundqvist, by guest on November 20, 2016 (Co-Chair)12 REVIEWERS: Bulent Gorenek, (review coordinator)17, Nikolaos Dagres4, Gheorge-Andrei Dan18, Marc A Vos19, Gulmira Kudaiberdieva20, Harry Crijns21, Kurt Roberts-Thomson22, Yenn-Jiang Lin23, Diego Vanegas24, Walter Reyes Caorsi25, Edmond Cronin26, and Jack Rickard27 1Athens Euroclinic, Athens, Greece; and Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, USA; 2Cardiology Department, Modena University Hospital, University of Modena and Reggio Emilia, Modena, Italy; 3Hospital Universitario De Getafe, Madrid, Spain; 4University of Leipzig, Heartcenter, Leipzig, Germany; 5University of Bordeaux, CHU Bordeaux, LIRYC, France; 6Berth Israel Deaconess Medical Center, Boston, MA, USA; 7Hospital Privado del Sur y Hospital Espan˜ol, Bahia -

JUNCTIONAL RHYTHMS and DYSRHYTHMIAS Connie J
Northwest Community Healthcare Paramedic Program JUNCTIONAL DYSRHYTHMIAS Connie J. Mattera, M.S., R.N., EMT-P Reading assignments: Bledsoe Vol 3: pp. 93-96 SOP: Bradycardia with a pulse KNOWLEDGE OBJECTIVES: Upon completion of the reading assignments, class, and homework questions, reviewing the SOPs, and working with their small group, each participant will independently do the following with at least an 80% degree of accuracy and no critical errors: 1. Identify on a 6-second strip the following rhythms: a) Junctional rhythm b) Accelerated junctional rhythm c) Junctional tachycardia d) Junctional escape beats e) Premature Junctional Contractions (PJCs) 2. Systematically evaluate each rhythm using the following criteria: a) Rate b) Rhythm: Regular/irregular c) Presence/absence/morphology of P waves d) R-R Interval, P-P Interval e) P-QRS relationship f) QRS duration 3. Correlate the cardiac rhythm with patient assessment findings to determine the EMS treatment for each rhythm according to NWC EMSS SOPs. 4. Discuss the action, prehospital indications, contraindications, dose, route, and side effects of the following: a) Atropine b) Norepinephrine (Dopamine backup alternate drug) c) Glucagon 5. Explain the indications, contraindications, process steps, and patient monitoring priorities when performing transcutaneous pacing (TCP). CJM: F06: 12/10; 11/12; 12/13; 12/14; 12/15; 11/16; 11/17 NCH Paramedic Program JUNCTIONAL RHYTHMS and DYSRHYTHMIAS Connie J. Mattera, M.S., R.N., EMT-P I. AV Junction as a pacemaker A. Etiology 1. The AV node (junction) can function as the heart’s pacemaker or it may initiate an isolated escape (late) beat when the sinus node fails to fire on time, or it may trigger early ectopic beats (PJCs). -

1 Natural History of Coagulopathy and Use Of
NATURAL HISTORY OF COAGULOPATHY AND USE OF ANTI-THROMBOTIC AGENTS IN COVID-19 PATIENTS AND PERSONS VACCINATED AGAINST SARS-COV-2 Principal Investigators Prof Dani Prieto-Alhambra (University of Oxford) Associate Prof Katia Verhamme (EMC) Associate Prof Peter Rijnbeek (EMC) Document Status Date of final version of the study Protocol ver 1.0 report EU PAS register number EUPAS40414 1 PASS information Title Natural history of coagulopathy and use of anti-thrombotic agents in COVID-19 patients and persons vaccinated against SARS-CoV-2 Protocol version identifier 1.0 Date of last version of protocol 12/04/2021 EU PAS register number EUPAS40414 Active Ingredient n/a Medicinal product J07BX Product reference n/a Procedure number n/a Marketing authorisation holder(s) n/a Joint PASS n/a Research question and objectives 1) To estimate the background incidence of selected embolic and thrombotic events of interest among the general population. 2) To estimate the incidence of selected embolic and thrombotic events of interest among persons vaccinated against SARS-CoV-2 at 7, 14, 21, and 28 days. 3) To estimate incidence rate ratios for selected embolic/thrombotic events of interest amongst people vaccinated against SARS-CoV-2 compared to background rates as estimated in Objective #1. 4) To estimate the incidence of venous thromboembolic events among patients with COVID-19 at 30-, 60-, and 90-days. 5) To calculate the risks of COVID-19 worsening stratified by the occurrence of a venous thromboembolic event. 6) To assess the impact of risk factors on the rates of venous thromboembolic events among patients with COVID-19. -

Advanced EKG Interpretation JUNCTIONAL RHYTHMS and NURSING INTERVENTIONS Objectives
Advanced EKG Interpretation JUNCTIONAL RHYTHMS AND NURSING INTERVENTIONS Objectives ♥ Identify specific cardiac dysrhythmias ♥ Describe appropriate nursing interventions for specific dysrhythmias Junctional Rhythms ▪ Junctional rhythms are named such because their impulse originates from the AV node (AV junction) instead of the SA node. ▪ The SA node may be impaired secondary to drug toxicity or underlying cardiac disease. ▪ When the AV node does not sense an impulse coming down from the SA node, it will become the pacemaker of the heart. Characteristics of all Junctional Rhythms ▪ Inverted (negative) or absent P waves are seen before each QRS complex OR ▪ P wave can be hidden in the QRS complex OR ▪ P wave may follow the QRS complex ▪ PR interval of <0.12 seconds (remember normal is 0.12-0.2) ▪ QRS complex within normal measurements Most Common Variations ▪ Junctional (escape) rhythm: 40 - 60 bpm ▪ Accelerated junctional rhythm: 61 – 100 bpm ▪ Junctional tachycardia: >100 bpm ▪ Premature junctional complexes (PJCs) Junctional Rhythm ♥ Junctional (escape) rhythms originate at or around the AV node and the Bundle of His. The impulse travels up the atria and down to the ventricles resulting in inverted P waves that can occur prior to, during or after the QRS. ♥ P waves can also be absent if the impulse does not travel up into the atria. Inverted P wave 5 Steps to Identify Junctional Rhythm 1. What is the rate? 40-60 bpm 2. What is the rhythm? Regular 3. Is there a P wave before each QRS? Are P waves upright Usually inverted or absent, may be before, during or after and uniform? QRS complex 4. -

(12) United States Patent (10) Patent No.: US 8,980,630 B2 Woodbury Et Al
USOO898063 OB2 (12) United States Patent (10) Patent No.: US 8,980,630 B2 Woodbury et al. (45) Date of Patent: Mar. 17, 2015 (54) OBTAINING MULTIPOTENT (56) References Cited AMNION-DERIVED STEM CELL (ADSC) U.S. PATENT DOCUMENTS FROM AMNOTIC MEMBRANE TISSUE M WITHOUT ENZYMATIC DIGESTION 2005, 0118712 A1 6, 2005 Tsai et al. 2007/0243172 A1 10, 2007 Ra et al. (75) Inventors: Dale Woodbury, Middletown, NJ (US); Akiva J. Marcus, Teaneck, NJ (US) FOREIGN PATENT DOCUMENTS (73) Assignee: Rutgers, The State University of New WO WOOOf73421 * 12/2000 WO O2O64.755 A2 8, 2002 Jersey, Somerset, NJ (US) WO 2005042703 A2 5, 2005 WO 2006O19357 A1 2, 2006 (*) Notice: Subject to any disclaimer, the term of this patent is extended or adjusted under 35 OTHER PUBLICATIONS U.S.C. 154(b) by 1293 days. Zhao et al. Human Amniotic Mesenchymal Cells Have Some Char acteristics of Cardiomyocytes Transplantation, Mar. 2005, vol. 79, (21) Appl. No.: 12/306,871 pp. 528-535.* Tsai et al. Isolation of human multipotent mesenchymal stem cells (22) PCT Filed: Jun. 28, 2007 from second-trimester amniotic fluid using a novel two-stage culture protocol Human Reproduction, 2004, vol. 19, pp. 1450-1456.* (86). PCT No.: PCT/US2007/072356 notypessgyet of Neuroglial al. As Progenior style Uells. J Neuroscience Cells E.s: Kes. S371 (c)(1), vol. 78, pp. 208-214.* (2), (4) Date: May 6, 2009 Grueterichet al. Connexin 43 Expression and Proliferation of Human Limbal Epithelium on Intact and Denuded Amniotic Membrane. (87) PCT Pub. No.: WO2008/003042 Investigative Ophthalmology and VicSula Science, 2002, vol.43, pp. -

Banner Staff Service ECG Study Guide
Banner Staff Service ECG Study Guide Edited by Larry H. Lybbert, MS, RN Table of Contents ECG STUDY GUIDE ................................................................................................................................................... 3 ECG INTERPRETATION BASICS........................................................................................................................... 4 EKG GRAPH PAPER ..................................................................................................................................................4 RATE MEASUREMENT...............................................................................................................................................9 The Six Second Method........................................................................................................................................9 Large Box Method................................................................................................................................................9 Small Box Method ................................................................................................................................................9 BASIC EKG RHYTHM ANALYSIS GUIDE ................................................................................................................11 Rhythm Interpretation........................................................................................................................................13 Six Basic Steps for Rhythm Interpretation -

Guidelines for the Interpretation of the Neonatal Electrocardiogram
European Heart Journal (2002) 23, 1329–1344 doi:10.1053/euhj.2002.3274, available online at http://www.idealibrary.com on Task Force Report Guidelines for the interpretation of the neonatal electrocardiogram A Task Force of the European Society of Cardiology P. J. Schwartz1 (Chair), A. Garson, Jr2, T. Paul3, M. Stramba-Badiale4, V. L. Vetter5, E. Villain6 and C. Wren7 1Department of Cardiology, University of Pavia and IRCCS Policlinico S. Matteo, Pavia, Italy; 2University of Virginia, Charlottesville, VA, U.S.A.; 3The Children’s Heart Program of South Carolina, Medical University of South Carolina, Charleston, SC, U.S.A.; 4Pediatric Arrhythmias Center, IRCCS Istituto Auxologico Italiano, Milan, Italy; 5Division of Pediatric Cardiology, Department of Pediatrics, Children’s Hospital of Philadelphia, University of Pennsylvania School of Medicine, Philadelphia, PA, U.S.A.; 6Division of Pediatric Cardiology, Department of Pediatrics, Hoˆpital Necker Enfants Malades, Paris, France; 7Department of Paediatric Cardiology, Freeman Hospital, Newcastle upon Tyne, U.K. Introduction.............................................................1329 Left ventricular hypertrophy............................1338 Normal electrocardiogram in the newborn .............1330 Low QRS voltage.............................................1338 Normal values......................................................1330 Ventricular repolarization....................................1338 Technology ..........................................................1330 QT prolongation: -
Hyperkalemia-Induced Coronary Artery Spasm and Junctional Tachycardia in Diabetic Ketoacidosis Reversed with Insulin and Saline, a Case Report
Global Journal of Anesthesia & Pain Medicine DOI: 10.32474/GJAPM.2020.02.000149 ISSN: 2644-1403 Case Report Hyperkalemia-Induced Coronary Artery Spasm and Junctional Tachycardia in Diabetic Ketoacidosis Reversed with Insulin and Saline, A Case Report Yasser Mohammed Hassanain Elsayed* Critical Care Unit, Fraskour Central Hospital, Egypt *Corresponding author: Critical Care Unit, Fraskour Central Hospital, Damietta Health Affairs, Egyptian Ministry of Health (MOH), Damietta, Egypt Received: February 11, 2020 Published: February 25, 2020 Abstract Rationale: Serum potassium concentration is usually elevated in the cases of diabetic ketoacidosis. Coronary artery spasm is recognized after the hematological chemical disturbance. Hyperkalemia is a rare cause of junctional tachycardia. Insulin decreases potassium levels in the blood by redistributing it into cells via increased sodium-potassium pump activity. Patient concerns: A young housewife female patient presented to the emergency department with diabetic ketoacidosis, coronary artery spasm, and junctional tachycardia. Diagnosis: Hyperkalemia-induced coronary artery spasm and junctional tachycardia in diabetic ketoacidosis. Interventions: Electrocardiography, oxygenation, central venous pressure monitoring, and echocardiography. Lessons: Hyperkalemia is a possible cause for both coronary artery spasm and junctional tachycardia in diabetic ketoacidosis. Electrolytes disturbance especially hyperkalemia is a significant serious metabolic problem in ketoacidosis. Outcomes: Successful reversal