Arrhythmias in Children
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Rhythms & Cardiac Emergencies
Rhythm & 12 Lead EKG Review March 2011 CE Condell Medical Center EMS System Site code # 107200E-1211 Prepared by: FF/PMD Michael Mounts – Lake Forest Fire Revised By: Sharon Hopkins, RN, BSN, EMT-P Objectives Upon successful completion of this module, the EMS provider will be able to: • Identify the components of a rhythm strip • Identify what the components represent on the rhythm strip • Identify criteria for sinus rhythms • Identify criteria for atrial rhythms • Identify AV/junctional rhythms Objectives cont. • Identify ventricular rhythms • Identify rhythms with AV blocks • Identify treatments for different rhythms • Identify criteria for identification of ST elevation on 12 lead EKG’s • Identify EMS treatment for patients with acute coronary syndrome (ST elevation) • Demonstrate standard & alternate placement of ECG electrodes for monitoring Objectives cont. • Demonstrate placement of electrodes for obtaining a 12 lead EKG • Demonstrate the ability to identify a variety of static or dynamic EKG rhythm strips • Demonstrate the ability to identify the presence or absence of ST elevation when presented with a 12 lead EKG • Review department’s process to transmit 12 lead EKG to hospital, if capable • Successfully complete the post quiz with a score of 80% or better. ECG Paper • What do the boxes represent? • How do you measure time & amplitude? Components of the Rhythm Strip • ECG Paper • Wave forms • Wave complexes • Wave segments • Wave intervals Wave Forms, Complexes, Segments & Intervals • P wave – atrial depolarization • QRS – Ventricular -

View Pdf Copy of Original Document
Phenotype definition for the Vanderbilt Genome-Electronic Records project Identifying genetics determinants of normal QRS duration (QRSd) Patient population: • Patients with DNA whose first electrocardiogram (ECG) is designated as “normal” and lacking an exclusion criteria. • For this study, case and control are drawn from the same population and analyzed via continuous trait analysis. The only difference will be the QRSd. Hypothetical timeline for a single patient: Notes: • The study ECG is the first normal ECG. • The “Mildly abnormal” ECG cannot be abnormal by presence of heart disease. It can have abnormal rate, be recorded in the presence of Na-channel blocking meds, etc. For instance, a HR >100 is OK but not a bundle branch block. • Y duration = from first entry in the electronic medical record (EMR) until one month following normal ECG • Z duration = most recent clinic visit or problem list (if present) to one week following the normal ECG. Labs values, though, must be +/- 48h from the ECG time Criteria to be included in the analysis: Criteria Source/Method “Normal” ECG must be: • QRSd between 65-120ms ECG calculations • ECG designed as “NORMAL” ECG classification • Heart Rate between 50-100 ECG calculations • ECG Impression must not contain Natural Language Processing (NLP) on evidence of heart disease concepts (see ECG impression. Will exclude all but list below) negated terms (e.g., exclude those with possible, probable, or asserted bundle branch blocks). Should also exclude normalization negations like “LBBB no longer present.” -

Sinus Node Ischemia—A Unique Presentation
International Journal of Clinical Medicine, 2015, 6, 50-54 Published Online January 2015 in SciRes. http://www.scirp.org/journal/ijcm http://dx.doi.org/10.4236/ijcm.2015.61007 Sinus Node Ischemia—A Unique Presentation Jwalit Morakhia1, Padmakumar Ramachandran1, Naveen Chandra Ganiga Sanjeeva1, Harikrishna Damodaran2, Shivani Kothari3, Ashok Thakkar3 1Department of Cardiology, Kasturba Medical College & Hospital, Karnataka, India 2Department of Cardiology, Pariyaram Medical College, Kerala, India 3Department of Clinical Trials, Sahajanand Medical Technologies Pvt. Ltd., Gujarat, India Email: [email protected] Received 27 December 2014; accepted 13 January 2015; published 20 January 2015 Copyright © 2015 by authors and Scientific Research Publishing Inc. This work is licensed under the Creative Commons Attribution International License (CC BY). http://creativecommons.org/licenses/by/4.0/ Abstract Sinus node dysfunction, as the sole manifestation of an acute coronary syndrome, is rare. We re- port a case of ischemic dysfunction of the sinus node in a patient who had previously undergone coronary artery bypass grafting for triple vessel disease. Intermittent rest angina with a junctional rhythm was noted in spite of patent grafts to all three vessels, which resolved after percutaneous revascularization of the right coronary artery. Keywords Sinus Node, Angina, Acute Coronary Syndrome, Thrombus Aspiration 1. Introduction Patients presenting with acute coronary syndromes, with atypical symptoms, are frequently misdiagnosed and under-treated. Embolic phenomena are sometimes overlooked by interventionalists as percutaneous coronary intervention is a specialty which primarily focuses on stenosis and occlusions [1]. We report a case of reversible sinus node dysfunction in the unique setting of post-coronary artery bypass grafting (CABG) status with patent grafts. -

An Extremely Rare Cause of Wolff-Parkinson
108 Erciyes Med J 2019; 41(1): 108–10 • DOI: 10.14744/etd.2018.18165 An Extremely Rare Cause of Wolff-Parkinson-White Syndrome: Rhabdomyoma in Association With Tuberous Sclerosis CASE REPORT Özlem Elkıran , Cemşit Karakurt , Damla İnce ABSTRACT Rhabdomyomas are the most common primary cardiac tumors in infants and children. They are usually associated with tuberous sclerosis (TS). As the tumors tend to regress spontaneously, surgical intervention is not usually performed unless they become obstructive or cause incessant arrhythmias. We report an extremely rare case of rhabdomyoma serving as a substrate for Wolff-Parkinson-White (WPW) syndrome and intractable supraventricular tachycardia accompanied by TS. Our case is particularly interesting because it was diagnosed prenatally. The signs of WPW syndrome disappeared from the elec- trocardiogram with the regression of the tumor. Keywords: Wolff-Parkinson-White Syndrome, child, rhabdomyoma INTRODUCTION Rhabdomyomas are the most common cardiac tumors in infants and children, and they are closely related with tuberous sclerosis (TS). A significant part of rhabdomyomas is asymptomatic, and they regress on follow-up. However, symptoms of cardiac failure, arrhythmias, and obstruction can be observed depending on their location in the heart. They require urgent medical or surgical treatment (1, 2). Cite this article as: Elkıran Ö, Karakurt C, İnce D. An Extremely Rhabdomyoma-related arrhythmias are reported as premature atrial and ventricular contractions, supraventricular Rare Cause of and ventricular tachycardia, sinus node dysfunction, atrioventricular block, and Wolff-Parkinson-White (WPW) Wolff-Parkinson-White syndrome (1, 3, 4). There are only a few studies of WPW syndrome occurring in association with TS, with and Syndrome: Rhabdomyoma in Association With without rhabdomyoma. -
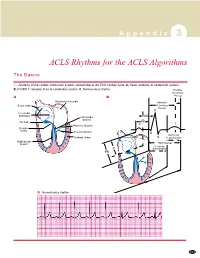
ACLS Rhythms for the ACLS Algorithms
A p p e n d i x 3 ACLS Rhythms for the ACLS Algorithms The Basics 1. Anatomy of the cardiac conduction system: relationship to the ECG cardiac cycle. A, Heart: anatomy of conduction system. B, P-QRS-T complex: lines to conduction system. C, Normal sinus rhythm. Relative Refractory A B Period Bachmann’s bundle Absolute Sinus node Refractory Period R Internodal pathways Left bundle AVN branch AV node PR T Posterior division P Bundle of His Anterior division Q Ventricular Purkinje fibers S Repolarization Right bundle branch QT Interval Ventricular P Depolarization PR C Normal sinus rhythm 253 A p p e n d i x 3 The Cardiac Arrest Rhythms 2. Ventricular Fibrillation/Pulseless Ventricular Tachycardia Pathophysiology ■ Ventricles consist of areas of normal myocardium alternating with areas of ischemic, injured, or infarcted myocardium, leading to chaotic pattern of ventricular depolarization Defining Criteria per ECG ■ Rate/QRS complex: unable to determine; no recognizable P, QRS, or T waves ■ Rhythm: indeterminate; pattern of sharp up (peak) and down (trough) deflections ■ Amplitude: measured from peak-to-trough; often used subjectively to describe VF as fine (peak-to- trough 2 to <5 mm), medium-moderate (5 to <10 mm), coarse (10 to <15 mm), very coarse (>15 mm) Clinical Manifestations ■ Pulse disappears with onset of VF ■ Collapse, unconsciousness ■ Agonal breaths ➔ apnea in <5 min ■ Onset of reversible death Common Etiologies ■ Acute coronary syndromes leading to ischemic areas of myocardium ■ Stable-to-unstable VT, untreated ■ PVCs with -

Dysrhythmias
CARDIOVASCULAR DISORDERS DYSRHYTHMIAS I. BASIC PRINCIPLES OF CARDIAC CONDUCTION DISTURBANCES A. Standard ECG and rhythm strips 1. Recordings are obtained at a paper speed of 25 mm/sec. 2. The vertical axis measures distance; the smallest divisions are 1 mm ×1 mm. 3. The horizontal axis measures time; each small division is 0.04 sec/mm. B. Normal morphology Courtesy of Dr. Michael McCrea 1. P wave = atrial depolarization a. Upright in leads I, II, III, aVL, and aVF; inverted in lead aVR b. Measures <0.10 seconds wide and <3 mm high c. Normal PR interval is 0.12–0.20 seconds. 2. QRS complex = ventricular depolarization a. Measures 0.06-0.10 seconds wide b. Q wave (1) <0.04 seconds wide and <3 mm deep (2) Abnormal if it is >3 mm deep or >1/3 of the QRS complex. c. R wave ≤7.5 mm high 3. QT interval varies with rate and sex but is usually 0.33–0.42 seconds; at normal heart rates, it is normally <1/2 the preceding RR interval. 4. T wave = ventricular repolarization a. Upright in leads I, II, V3–V6; inverted in aVR b. Slightly rounded and asymmetric in configuration c. Measures ≤5 mm high in limb leads and ≤10 mm high in the chest leads 5. U wave = a ventricular afterpotential a. Any deflection after the T wave (usually low voltage) b. Same polarity as the T wave c. Most easily detected in lead V3 d. Can be a normal component of the ECG e. Prominent U waves may indicate one of the following: (1) Hypokalemia (<3 mEq/L) (2) Hypercalcemia (3) Therapy with digitalis, phenothiazines, quinidine, epinephrine, inotropic agents, or amiodarone (4) Thyrotoxicosis f. -
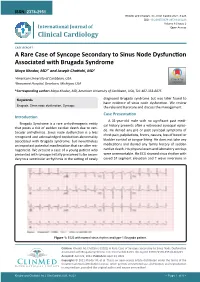
A Rare Case of Syncope Secondary to Sinus Node Dysfunction Associated with Brugada Syndrome Maya Khodor, MD1* and Joseph Chattahi, MD2
ISSN: 2378-2951 Khodor and Chattahi. Int J Clin Cardiol 2021, 8:223 DOI: 10.23937/2378-2951/1410223 Volume 8 | Issue 2 International Journal of Open Access Clinical Cardiology CASE REPORT A Rare Case of Syncope Secondary to Sinus Node Dysfunction Associated with Brugada Syndrome Maya Khodor, MD1* and Joseph Chattahi, MD2 1 American University of Caribbean, USA Check for 2Beaumont Hospital, Dearborn, Michigan, USA updates *Corresponding author: Maya Khodor, MD, American University of Caribbean, USA, Tel: 407-353-8875 diagnosed Brugada syndrome but was later found to Keywords have evidence of sinus node dysfunction. We review Brugada, Sinus node dysfunction, Syncope the relevant literature and discuss the management. Case Presentation Introduction A 31-year-old male with no significant past medi- Brugada Syndrome is a rare arrhythmogenic entity cal history presents after a witnessed syncopal episo- that poses a risk of sudden cardiac death due to ven- de. He denied any pre or post syncopal symptoms of tricular arrhythmias. Sinus node dysfunction is a less chest pain, palpitations, fevers, nausea, loss of bowel or recognized and acknowledged conduction abnormality associated with Brugada syndrome, but nevertheless bladder control or tongue biting. He does not take any an important potential manifestation that can alter ma- medications and denied any family history of sudden nagement. We present a case of a young patient who cardiac death. His physical exam and laboratory workup presented with syncope initially presumed to be secon- were unremarkable. His ECG showed sinus rhythm with dary to a ventricular arrhythmia in the setting of newly coved ST segment elevation and T wave inversions in Figure 1: ECG with normal sinus rhythm and type-1 Brugada pattern. -

(EHRA) Consensus Document on the Management of Supraventricular Arrhythmias, Endorsed by Heart
Europace Advance Access published November 17, 2016 Europace EHRA POSITION PAPER doi:10.1093/europace/euw301 European Heart Rhythm Association (EHRA) consensus document on the management of supraventricular arrhythmias, endorsed by Heart Rhythm Society (HRS), Asia-Pacific Heart Rhythm Society (APHRS), and Sociedad Latinoamericana de Estimulacio´nCardiacay Electrofisiologia (SOLAECE) Demosthenes G. Katritsis, (Chair)1, Giuseppe Boriani2,FranciscoG.Cosio3, Gerhard Hindricks4,PierreJaı¨s5,MarkE.Josephson6, Roberto Keegan7, Young-Hoon Kim8, Bradley P. Knight9,Karl-HeinzKuck10,DeirdreA.Lane10,11, Downloaded from GregoryY.H.Lip11, Helena Malmborg12, Hakan Oral13, Carlo Pappone14, 15 16 Sakis Themistoclakis ,KathrynA.Wood , and Carina Blomstro¨m-Lundqvist, by guest on November 20, 2016 (Co-Chair)12 REVIEWERS: Bulent Gorenek, (review coordinator)17, Nikolaos Dagres4, Gheorge-Andrei Dan18, Marc A Vos19, Gulmira Kudaiberdieva20, Harry Crijns21, Kurt Roberts-Thomson22, Yenn-Jiang Lin23, Diego Vanegas24, Walter Reyes Caorsi25, Edmond Cronin26, and Jack Rickard27 1Athens Euroclinic, Athens, Greece; and Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, USA; 2Cardiology Department, Modena University Hospital, University of Modena and Reggio Emilia, Modena, Italy; 3Hospital Universitario De Getafe, Madrid, Spain; 4University of Leipzig, Heartcenter, Leipzig, Germany; 5University of Bordeaux, CHU Bordeaux, LIRYC, France; 6Berth Israel Deaconess Medical Center, Boston, MA, USA; 7Hospital Privado del Sur y Hospital Espan˜ol, Bahia -

JUNCTIONAL RHYTHMS and DYSRHYTHMIAS Connie J
Northwest Community Healthcare Paramedic Program JUNCTIONAL DYSRHYTHMIAS Connie J. Mattera, M.S., R.N., EMT-P Reading assignments: Bledsoe Vol 3: pp. 93-96 SOP: Bradycardia with a pulse KNOWLEDGE OBJECTIVES: Upon completion of the reading assignments, class, and homework questions, reviewing the SOPs, and working with their small group, each participant will independently do the following with at least an 80% degree of accuracy and no critical errors: 1. Identify on a 6-second strip the following rhythms: a) Junctional rhythm b) Accelerated junctional rhythm c) Junctional tachycardia d) Junctional escape beats e) Premature Junctional Contractions (PJCs) 2. Systematically evaluate each rhythm using the following criteria: a) Rate b) Rhythm: Regular/irregular c) Presence/absence/morphology of P waves d) R-R Interval, P-P Interval e) P-QRS relationship f) QRS duration 3. Correlate the cardiac rhythm with patient assessment findings to determine the EMS treatment for each rhythm according to NWC EMSS SOPs. 4. Discuss the action, prehospital indications, contraindications, dose, route, and side effects of the following: a) Atropine b) Norepinephrine (Dopamine backup alternate drug) c) Glucagon 5. Explain the indications, contraindications, process steps, and patient monitoring priorities when performing transcutaneous pacing (TCP). CJM: F06: 12/10; 11/12; 12/13; 12/14; 12/15; 11/16; 11/17 NCH Paramedic Program JUNCTIONAL RHYTHMS and DYSRHYTHMIAS Connie J. Mattera, M.S., R.N., EMT-P I. AV Junction as a pacemaker A. Etiology 1. The AV node (junction) can function as the heart’s pacemaker or it may initiate an isolated escape (late) beat when the sinus node fails to fire on time, or it may trigger early ectopic beats (PJCs). -

Sick Sinus Syndrome After the Maze Procedure Performed
ORIGINAL RESEARCH Sick Sinus Syndrome After the Maze Procedure Performed Concomitantly With Mitral Valve Surgery Min Soo Cho, MD; Ran Heo, MD; Xin Jin, MD; Jung-Bok Lee, PhD; Sahmin Lee, MD, PhD; Dae-Hee Kim, MD, PhD; Joon Bum Kim, MD, PhD; Jun Kim, MD, PhD; Sung-Ho Jung, MD, PhD; Suk Jung Choo, MD, PhD; Jong-Min Song, MD, PhD; Gi-Byoung Nam, MD, PhD; Kee-Joon Choi, MD, PhD; Duk-Hyun Kang, MD, PhD; Cheol Hyun Chung, MD, PhD; Jae Won Lee, MD, PhD; You-Ho Kim, MD, PhD; Jae-Kwan Song, MD, PhD Background-—To characterize the development of sick sinus syndrome (SSS) after the additive maze procedure (MP) during mitral valve surgery. Methods and Results-—Follow-up data (median, 3.6 years) of 750 patients with a prevalence of rheumatic cause of 57.6% were analyzed. SSS occurred in 35 patients with a time-dependent increase: the incidence rates at 1, 2, and 4 years after surgery were 2.9%, 3.7%, and 4.3%, respectively. The additive MP showed higher risks of SSS development (hazard ratio, 7.44; 95% confidence interval, 3.45–16.05; P<0.001) and pacemaker implantation (hazard ratio, 3.61; 95% confidence interval, 1.95–6.67; P<0.001). Patients who developed SSS showed higher 4-year rates of clinical events (death, stroke, and hospital admission) (67.5Æ8.5% versus 33.0Æ1.9%; P<0.001). After adjustment for age and preoperative peak systolic pulmonary artery pressure, the lesion extent (biatrial versus left atrial MP), not the underlying cause (rheumatic versus nonrheumatic), was independently associated with SSS development (hazard ratio, 3.58; 95% confidence interval, 1.08–11.86; P=0.037). -

1 Natural History of Coagulopathy and Use Of
NATURAL HISTORY OF COAGULOPATHY AND USE OF ANTI-THROMBOTIC AGENTS IN COVID-19 PATIENTS AND PERSONS VACCINATED AGAINST SARS-COV-2 Principal Investigators Prof Dani Prieto-Alhambra (University of Oxford) Associate Prof Katia Verhamme (EMC) Associate Prof Peter Rijnbeek (EMC) Document Status Date of final version of the study Protocol ver 1.0 report EU PAS register number EUPAS40414 1 PASS information Title Natural history of coagulopathy and use of anti-thrombotic agents in COVID-19 patients and persons vaccinated against SARS-CoV-2 Protocol version identifier 1.0 Date of last version of protocol 12/04/2021 EU PAS register number EUPAS40414 Active Ingredient n/a Medicinal product J07BX Product reference n/a Procedure number n/a Marketing authorisation holder(s) n/a Joint PASS n/a Research question and objectives 1) To estimate the background incidence of selected embolic and thrombotic events of interest among the general population. 2) To estimate the incidence of selected embolic and thrombotic events of interest among persons vaccinated against SARS-CoV-2 at 7, 14, 21, and 28 days. 3) To estimate incidence rate ratios for selected embolic/thrombotic events of interest amongst people vaccinated against SARS-CoV-2 compared to background rates as estimated in Objective #1. 4) To estimate the incidence of venous thromboembolic events among patients with COVID-19 at 30-, 60-, and 90-days. 5) To calculate the risks of COVID-19 worsening stratified by the occurrence of a venous thromboembolic event. 6) To assess the impact of risk factors on the rates of venous thromboembolic events among patients with COVID-19. -

Management of Asymptomatic Arrhythmias
Europace (2019) 0, 1–32 EHRA POSITION PAPER doi:10.1093/europace/euz046 Downloaded from https://academic.oup.com/europace/advance-article-abstract/doi/10.1093/europace/euz046/5382236 by PPD Development LP user on 25 April 2019 Management of asymptomatic arrhythmias: a European Heart Rhythm Association (EHRA) consensus document, endorsed by the Heart Failure Association (HFA), Heart Rhythm Society (HRS), Asia Pacific Heart Rhythm Society (APHRS), Cardiac Arrhythmia Society of Southern Africa (CASSA), and Latin America Heart Rhythm Society (LAHRS) David O. Arnar (Iceland, Chair)1*, Georges H. Mairesse (Belgium, Co-Chair)2, Giuseppe Boriani (Italy)3, Hugh Calkins (USA, HRS representative)4, Ashley Chin (South Africa, CASSA representative)5, Andrew Coats (United Kingdom, HFA representative)6, Jean-Claude Deharo (France)7, Jesper Hastrup Svendsen (Denmark)8,9, Hein Heidbu¨chel (Belgium)10, Rodrigo Isa (Chile, LAHRS representative)11, Jonathan M. Kalman (Australia, APHRS representative)12,13, Deirdre A. Lane (United Kingdom)14,15, Ruan Louw (South Africa, CASSA representative)16, Gregory Y. H. Lip (United Kingdom, Denmark)14,15, Philippe Maury (France)17, Tatjana Potpara (Serbia)18, Frederic Sacher (France)19, Prashanthan Sanders (Australia, APHRS representative)20, Niraj Varma (USA, HRS representative)21, and Laurent Fauchier (France)22 ESC Scientific Document Group: Kristina Haugaa23,24, Peter Schwartz25, Andrea Sarkozy26, Sanjay Sharma27, Erik Kongsga˚rd28, Anneli Svensson29, Radoslaw Lenarczyk30, Maurizio Volterrani31, Mintu Turakhia32,