Ch 5. Molecules and Compounds Chemical Formulas of Compounds 1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Tutorial 2 FORMULAS, PERCENTAGE COMPOSITION
T-6 Tutorial 2 FORMULAS, PERCENTAGE COMPOSITION, AND THE MOLE FORMULAS: A chemical formula shows the elemental composition of a substance: the chemical symbols show what elements are present and the numerical subscripts show how many atoms of each element there are in a formula unit. Examples: NaCl: one sodium atom, one chlorine atom in a formula unit CaCl2: one calcium atom, two chlorine atoms in a formula unit Mg3N2: three magnesium atoms, two nitrogen atoms in a formula unit The presence of a metal in a chemical formula indicates an ionic compound, which is composed of positive ions (cations) and negative ions (anions). A formula with only nonmetals indicates a + molecular compound (unless it is an ammonium, NH4 , compound). Only ionic compounds are considered in this Tutorial. There are tables of common ions in your lecture text, p 56 (cations) and p 57 (anions). A combined table of these same ions can be found on the inside back cover of the lecture text. A similar list is on the next page; all formulas needed in this and subsequent Tutorial problems can be written with ions from this list. Writing formulas for ionic compounds is very straightforward: TOTAL POSITIVE CHARGES MUST BE THE SAME AS TOTAL NEGATIVE CHARGES. The formula must be neutral. The positive ion is written first in the formula and the name of the compound is the two ion names. EXAMPLE: Write the formula for potassium chloride. The name tells you there are potassium, K+, and chloride, Cl–, ions. Each potassium ion is +1 and each chloride ion is -1: one of each is needed, and the formula for potassium chloride is KCl. -

Chemical Formula
Chemical Formula Jean Brainard, Ph.D. Say Thanks to the Authors Click http://www.ck12.org/saythanks (No sign in required) AUTHOR Jean Brainard, Ph.D. To access a customizable version of this book, as well as other interactive content, visit www.ck12.org CK-12 Foundation is a non-profit organization with a mission to reduce the cost of textbook materials for the K-12 market both in the U.S. and worldwide. Using an open-content, web-based collaborative model termed the FlexBook®, CK-12 intends to pioneer the generation and distribution of high-quality educational content that will serve both as core text as well as provide an adaptive environment for learning, powered through the FlexBook Platform®. Copyright © 2013 CK-12 Foundation, www.ck12.org The names “CK-12” and “CK12” and associated logos and the terms “FlexBook®” and “FlexBook Platform®” (collectively “CK-12 Marks”) are trademarks and service marks of CK-12 Foundation and are protected by federal, state, and international laws. Any form of reproduction of this book in any format or medium, in whole or in sections must include the referral attribution link http://www.ck12.org/saythanks (placed in a visible location) in addition to the following terms. Except as otherwise noted, all CK-12 Content (including CK-12 Curriculum Material) is made available to Users in accordance with the Creative Commons Attribution-Non-Commercial 3.0 Unported (CC BY-NC 3.0) License (http://creativecommons.org/ licenses/by-nc/3.0/), as amended and updated by Creative Com- mons from time to time (the “CC License”), which is incorporated herein by this reference. -

Presentation on Key Ideas of Elementary Mathematics
Key Ideas of Elementary Mathematics Sybilla Beckmann Department of Mathematics University of Georgia Lesson Study Conference, May 2007 Sybilla Beckmann (University of Georgia) Key Ideas of Elementary Mathematics 1/52 US curricula are unfocused A Splintered Vision, 1997 report based on the TIMSS curriculum analysis. US state math curriculum documents: “The planned coverage included so many topics that we cannot find a single, or even a few, major topics at any grade that are the focus of these curricular intentions. These official documents, individually or as a composite, are unfocused. They express policies, goals, and intended content coverage in mathematics and the sciences with little emphasis on particular, strategic topics.” Sybilla Beckmann (University of Georgia) Key Ideas of Elementary Mathematics 2/52 US instruction is unfocused From A Splintered Vision: “US eighth grade mathematics and science teachers typically teach far more topic areas than their counterparts in Germany and Japan.” “The five surveyed topic areas covered most extensively by US eighth grade mathematics teachers accounted for less than half of their year’s instructional periods. In contrast, the five most extensively covered Japanese eighth grade topic areas accounted for almost 75 percent of their year’s instructional periods.” Sybilla Beckmann (University of Georgia) Key Ideas of Elementary Mathematics 3/52 Breaking the “mile-wide-inch-deep” habit Every mathematical skill and concept has some useful application has some connection to other concepts and skills So what mathematics should we focus on? Sybilla Beckmann (University of Georgia) Key Ideas of Elementary Mathematics 4/52 What focus? Statistics and probability are increasingly important in science and in the modern workplace. -

2•Stoichiometry: Chemical Arithmetic Formula Conventions (1 Of
Superscripts used to show the charges on ions Mg2+ the 2 means a 2+ charge (lost 2 electrons) Subscripts 2•Stoichiometry: Chemical Arithmetic used to show numbers of atoms in a formula unit Formula Conventions H2SO4 two H’s, one S, and 4 O’s (1 of 24) Coefficients used to show the number of formula units 2Br– the 2 means two individual bromide ions Hydrates CuSO4 • 5 H2O some compounds have water molecules included stoichiometry study of the quantitative relationships in chemical formulas and equations. atomic mass weighted average mass of an atom, found on the periodic table 2•Stoichiometry: Chemical Arithmetic formula mass sum of the atomic masses of the Stoichiometry Terms atoms in a formula molecular mass sum of the atomic masses of the (2 of 24) atoms in a molecular formula gram molecular mass molecular mass written in grams molar mass same as gram molecular mass empirical formula formula reduced to lowest terms Formula or molecular mass is found by simply summing the atomic masses (on the periodic table) of each atom in a formula. H2SO4 2•Stoichiometry: Chemical Arithmetic 1.01 + 1.01 + 32.06 + 16.0 + 16.0 + 16.0 + 16.0 = 98.08 u Calculating Formula Mass 2(1.01) + 32.06 + 4(16.0) = 98.06 u or 98.06 g/mole (3 of 24) Generally, round off your answers to the hundredths or tenths place. Don’t round off too much (98.06 g/mol or 98.1 g/mol is OK, but don’t round off to 98 g/mol) Units Use u or amu if you are referring to one atom or molecule A mole (abbreviated mol) is a certain number of things. -

Solute Concentration: Molality
5/25/2012 Colligative Properties of Solutions . Colligative Properties: • Solution properties that depend on concentration of solute particles, not the identity of particles. Previous example: vapor pressure lowering. Consequences: change in b.p. and f.p. of solution. © 2012 by W. W. Norton & Company Solute Concentration: Molality . Changes in boiling point/freezing point of solutions depends on molality: moles of solute m kg of solvent • Preferred concentration unit for properties involving temperature changes because it is independent of temperature. © 2012 by W. W. Norton & Company 1 5/25/2012 Calculating Molality Starting with: a) Mass of solute and solvent. b) Mass of solute/ volume of solvent. c) Volume of solution. © 2012 by W. W. Norton & Company Sample Exercise 11.8 How many grams of Na2SO4 should be added to 275 mL of water to prepare a 0.750 m solution of Na2SO4? Assume the density of water is 1.000 g/mL. © 2012 by W. W. Norton & Company 2 5/25/2012 Boiling-Point Elevation and Freezing-Point Depression . Boiling Point Elevation (ΔTb): • ΔTb = Kb∙m • Kb = boiling point elevation constant of solvent; m = molality. Freezing Point Depression (ΔTf): • ΔTf = Kf∙m • Kf = freezing-point depression constant; m = molality. © 2012 by W. W. Norton & Company Sample Exercise 11.9 What is the boiling point of seawater if the concentration of ions in seawater is 1.15 m? © 2012 by W. W. Norton & Company 3 5/25/2012 Sample Exercise 11.10 What is the freezing point of radiator fluid prepared by mixing 1.00 L of ethylene glycol (HOCH2CH2OH, density 1.114 g/mL) with 1.00 L of water (density 1.000 g/mL)? The freezing-point-depression constant of water, Kf, is 1.86°C/m. -
Calculus Terminology
AP Calculus BC Calculus Terminology Absolute Convergence Asymptote Continued Sum Absolute Maximum Average Rate of Change Continuous Function Absolute Minimum Average Value of a Function Continuously Differentiable Function Absolutely Convergent Axis of Rotation Converge Acceleration Boundary Value Problem Converge Absolutely Alternating Series Bounded Function Converge Conditionally Alternating Series Remainder Bounded Sequence Convergence Tests Alternating Series Test Bounds of Integration Convergent Sequence Analytic Methods Calculus Convergent Series Annulus Cartesian Form Critical Number Antiderivative of a Function Cavalieri’s Principle Critical Point Approximation by Differentials Center of Mass Formula Critical Value Arc Length of a Curve Centroid Curly d Area below a Curve Chain Rule Curve Area between Curves Comparison Test Curve Sketching Area of an Ellipse Concave Cusp Area of a Parabolic Segment Concave Down Cylindrical Shell Method Area under a Curve Concave Up Decreasing Function Area Using Parametric Equations Conditional Convergence Definite Integral Area Using Polar Coordinates Constant Term Definite Integral Rules Degenerate Divergent Series Function Operations Del Operator e Fundamental Theorem of Calculus Deleted Neighborhood Ellipsoid GLB Derivative End Behavior Global Maximum Derivative of a Power Series Essential Discontinuity Global Minimum Derivative Rules Explicit Differentiation Golden Spiral Difference Quotient Explicit Function Graphic Methods Differentiable Exponential Decay Greatest Lower Bound Differential -
Unit 3: Chemistry Day 2 – Elements Vs
Unit 3: Chemistry Day 2 – Elements vs. Compounds Unit Essential Questions: Vocabulary: SWBAT 1. What is the smallest part of an element? Element … identify that a substance is a compound 2. How are atoms and elements related? Compound 3. How do scientists use density to identify by its chemical formula, absence on the substances? Chemical Formula Periodic Table, and that it contains two or Periodic Table of Elements more types of atoms. Video: Guiding Questions 1. How many elements need to combine in order to form a compound? 3. H2O is the chemical formula for what? 2. What is the recipe for a compound called? 4. What elements are present in water? 5. What is another compound the video talks about? GUIDED NOTES: Elements and Compounds Homework: Finish entire packet. STOP AND JOT: What is the difference between an element and a compound? Chemical Formulas Compounds are represented by _________________________________ _____________________________________. Each capital letter represents _______________ element. ____________________________________ represent the number of atoms of each element. Examples: H2O Si3N4 Number of elements: _____________________ Number of elements: _____________________ Number of atoms for each element: Number of atoms for each element: ______________________ ______________________ ______________________ ________________________ How many elements are in How many atoms of each How many elements are in How many atoms of each the compound H3PO4? element is present in the the compound C2H5OH? element is present in the compound, HNO3? compound, C2H4O2? Three ways to identify a Compound: 1. Made of more than one _________________________. 2. Not located on the _____________________________ __________________________ _________________ ________________________________. 3. Represented by a ____________________________________ ____________________________________. -

16.4 Calculations Involving Colligative Properties 16.4
chem_TE_ch16.fm Page 491 Tuesday, April 18, 2006 11:27 AM 16.4 Calculations Involving Colligative Properties 16.4 1 FOCUS Connecting to Your World Cooking instructions for a wide Guide for Reading variety of foods, from dried pasta to packaged beans to frozen fruits to Objectives fresh vegetables, often call for the addition of a small amount of salt to the Key Concepts • What are two ways of 16.4.1 Solve problems related to the cooking water. Most people like the flavor of expressing the concentration food cooked with salt. But adding salt can of a solution? molality and mole fraction of a have another effect on the cooking pro- • How are freezing-point solution cess. Recall that dissolved salt elevates depression and boiling-point elevation related to molality? 16.4.2 Describe how freezing-point the boiling point of water. Suppose you Vocabulary depression and boiling-point added a teaspoon of salt to two liters of molality (m) elevation are related to water. A teaspoon of salt has a mass of mole fraction molality. about 20 g. Would the resulting boiling molal freezing-point depression K point increase be enough to shorten constant ( f) the time required for cooking? In this molal boiling-point elevation Guide to Reading constant (K ) section, you will learn how to calculate the b amount the boiling point of the cooking Reading Strategy Build Vocabulary L2 water would rise. Before you read, make a list of the vocabulary terms above. As you Graphic Organizers Use a chart to read, write the symbols or formu- las that apply to each term and organize the definitions and the math- Molality and Mole Fraction describe the symbols or formulas ematical formulas associated with each using words. -

E.W. Dijkstra Archive: on the Cruelty of Really Teaching Computing Science
On the cruelty of really teaching computing science Edsger W. Dijkstra. (EWD1036) http://www.cs.utexas.edu/users/EWD/ewd10xx/EWD1036.PDF The second part of this talk pursues some of the scientific and educational consequences of the assumption that computers represent a radical novelty. In order to give this assumption clear contents, we have to be much more precise as to what we mean in this context by the adjective "radical". We shall do so in the first part of this talk, in which we shall furthermore supply evidence in support of our assumption. The usual way in which we plan today for tomorrow is in yesterday’s vocabulary. We do so, because we try to get away with the concepts we are familiar with and that have acquired their meanings in our past experience. Of course, the words and the concepts don’t quite fit because our future differs from our past, but then we stretch them a little bit. Linguists are quite familiar with the phenomenon that the meanings of words evolve over time, but also know that this is a slow and gradual process. It is the most common way of trying to cope with novelty: by means of metaphors and analogies we try to link the new to the old, the novel to the familiar. Under sufficiently slow and gradual change, it works reasonably well; in the case of a sharp discontinuity, however, the method breaks down: though we may glorify it with the name "common sense", our past experience is no longer relevant, the analogies become too shallow, and the metaphors become more misleading than illuminating. -
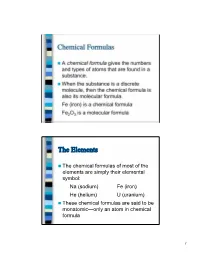
Chemical Formulas the Elements
Chemical Formulas A chemical formula gives the numbers and types of atoms that are found in a substance. When the substance is a discrete molecule, then the chemical formula is also its molecular formula. Fe (iron) is a chemical formula Fe2O3 is a molecular formula The Elements The chemical formulas of most of the elements are simply their elemental symbol: Na (sodium) Fe (iron) He (helium) U (uranium) These chemical formulas are said to be monatomic—only an atom in chemical formula 1 The Elements There are seven elements that occur naturally as diatomic molecules—molecules that contain two atoms: H2 (hydrogen) N2 (nitrogen) O2 (oxygen) F2 (fluorine) Cl2 (chlorine) Br2 (bromine) I2 (iodine) The last four elements in this list are in the same family of the Periodic Table Binary Compounds A binary compound is one composed of only two different types of atoms. Rules for binary compound formulas 1. Element to left in Periodic Table comes first except for hydrogen: KCl PCl3 Al2S3 Fe3O4 2 Binary Compounds 2. Hydrogen comes last unless other element is from group 16 or 17: LiH, NH3, B2H4, CH4 3. If both elements are from the same group, the lower element comes first: SiC, BrF3 Other Compounds For compounds with three or more elements that are not ionic, if it contains carbon, this comes first followed by hydrogen. Other elements are then listed in alphabetical order: C2H6O C4H9BrO CH3Cl C8H10N4O2 3 Other Compounds However, the preceding rule is often ignored when writing organic formulas (molecules containing carbon, hydrogen, and maybe other elements) in order to give a better idea of how the atoms are connected: C2H6O is the molecular formula for ethanol, but nobody ever writes it this way—instead the formula is written C2H5OH to indicate one H atom is connected to the O atom. -

Common Equations Used in Chemistry Equation For
Common Equations Used in Chemistry m Equation for density: d= v 5 Converting ˚F to ˚C: ˚C = (˚F - 32) x 9 9 Converting ˚C to ˚F: ˚F = ˚C x 5 + 32 Converting ˚C to K: K = (˚C + 273.15) n x molar mass of element Percent composition of an element = molar mass of compound x 100% - where n = the number of moles of the element in one mole of the compound actual yield % yield = theoretical yield x 100% moles of solute molarity (M) = liters of solution Dilution of Solution: MiVi = MfV f Boyle’s law - Constant T and n: PV = k Boyle’s law - For calculating changes in pressure or volume: P1V1 = P2V 2 V Charles’ law - Constant P and n: T = k V1 V 2 Charles’ law - For calculating temperature or volume changes: = T1 T2 Avogadro’s law - Constant P and T: V = kn Ideal Gas equation: PV = nRT Calculation of changes in pressure, temperature, or volume of gas when n is constant: P1V1 P2V 2 = T1 T2 PM Calculation of density or molar mass of gas: d = RT Dalton’s law of partial pressures - for calculating partial pressures: Pi = XiPT 3RT 0.5 Root-mean-square speed of gas molecules: urms = ( M ) Van der waals equation; for calculating the pressure of a nonideal gas: an 2 (P + ) (V - nb) = nRT V 2 Definition of heat capacity, where s is specific heat: C = ms Calculation of heat change in terms of specific heat : q = msDt Calculation of heat change in terms of heat capacity: q = CDt q1q2 Electrical force: F = k el r2 q1q2 Potential energy: V = k r Calculation of standard enthalpy of reaction: DH˚rxn = å nDH˚f (products) - å mDH˚f (reactants) [where n and m are coefficients in equation] Mathematical statement of the first law of thermodynamics: DE = q + w Work done in gas expansion or compression: w = - PDV Definition of enthalpy: H = E + PV Enthalpy (or energy) change for a constant-pressure process: DH = DE +PDV Enthalpy (or energy) change for a constant-pressure process: DE = DH - RTDn, where n is the change in the number of moles of gas. -

Zumdahl Chapter 5 Gases
5 Gases Contents 5.1 Pressure • Units of Pressure 5.2 The Gas Laws of Boyle, Charles, and Avogadro • Boyle’s Law • Charles’s Law • Avogadro’s Law 5.3 The Ideal Gas Law 5.4 Gas Stoichiometry • Molar Mass of a Gas 5.5 Dalton’s Law of Partial Pressures • Collecting a Gas over Water 5.6 The Kinetic Molecular Theory of Gases • Pressure and Volume (Boyle’s Law) • Pressure and Temperature • Volume and Temperature (Charles’s Law) • Volume and Number of Moles (Avogadro’s Law) • Mixture of Gases (Dalton’s Law) • Deriving the Ideal Gas Law • The Meaning of Temperature • Root Mean Square Velocity 5.7 Effusion and Diffusion • Effusion • Diffusion 5.8 Real Gases 5.9 Characteristics of Several Real Gases 5.10 Chemistry in the Atmosphere The steaming fumaroles located in Bjarnarflag, Iceland release a variety of gases. 178 Matter exists in three distinct physical states: gas, liquid, and solid. Although rel- atively few substances exist in the gaseous state under typical conditions, gases are very important. For example, we live immersed in a gaseous solution. The earth’s atmosphere is a mixture of gases that consists mainly of elemental nitrogen (N2) and oxygen (O2). The atmosphere both supports life and acts as a waste receptacle for the exhaust gases that accompany many industrial processes. The chemical reactions of these waste gases in the atmosphere lead to various types of pollution, including smog and acid rain. The gases in the atmosphere also shield us from harmful radiation from the sun and keep the earth warm by reflecting heat radiation back toward the earth.