Structure-Function Relationships in ABCG2: Insights from Molecular
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

ABCB6 Is a Porphyrin Transporter with a Novel Trafficking Signal That Is Conserved in Other ABC Transporters Yu Fukuda University of Tennessee Health Science Center
University of Tennessee Health Science Center UTHSC Digital Commons Theses and Dissertations (ETD) College of Graduate Health Sciences 12-2008 ABCB6 Is a Porphyrin Transporter with a Novel Trafficking Signal That Is Conserved in Other ABC Transporters Yu Fukuda University of Tennessee Health Science Center Follow this and additional works at: https://dc.uthsc.edu/dissertations Part of the Chemicals and Drugs Commons, and the Medical Sciences Commons Recommended Citation Fukuda, Yu , "ABCB6 Is a Porphyrin Transporter with a Novel Trafficking Signal That Is Conserved in Other ABC Transporters" (2008). Theses and Dissertations (ETD). Paper 345. http://dx.doi.org/10.21007/etd.cghs.2008.0100. This Dissertation is brought to you for free and open access by the College of Graduate Health Sciences at UTHSC Digital Commons. It has been accepted for inclusion in Theses and Dissertations (ETD) by an authorized administrator of UTHSC Digital Commons. For more information, please contact [email protected]. ABCB6 Is a Porphyrin Transporter with a Novel Trafficking Signal That Is Conserved in Other ABC Transporters Document Type Dissertation Degree Name Doctor of Philosophy (PhD) Program Interdisciplinary Program Research Advisor John D. Schuetz, Ph.D. Committee Linda Hendershot, Ph.D. James I. Morgan, Ph.D. Anjaparavanda P. Naren, Ph.D. Jie Zheng, Ph.D. DOI 10.21007/etd.cghs.2008.0100 This dissertation is available at UTHSC Digital Commons: https://dc.uthsc.edu/dissertations/345 ABCB6 IS A PORPHYRIN TRANSPORTER WITH A NOVEL TRAFFICKING SIGNAL THAT -

ABCB5 Recombinant Protein Cat
ABCB5 Recombinant Protein Cat. No.: 92-176 ABCB5 Recombinant Protein Specifications SPECIES: Human SOURCE SPECIES: E. coli SEQUENCE: Ile141-Val247 FUSION TAG: N-Trx tag TESTED APPLICATIONS: APPLICATIONS: This recombinant protein can be used for biological assays. For research use only. PREDICTED MOLECULAR 29.4 kD WEIGHT: Properties Greater than 95% as determined by reducing SDS-PAGE. PURITY: Endotoxin level less than 0.1 ng/ug (1 IEU/ug) as determined by LAL test. PHYSICAL STATE: Lyophilized Lyophilized from a 0.2 um filtered solution of 20mM PB,150mM NaCl,pH7.4. It is not BUFFER: recommended to reconstitute to a concentration less than 100 ug/ml. Dissolve the lyophilized protein in ddH2O. September 23, 2021 1 https://www.prosci-inc.com/abcb5-recombinant-protein-92-176.html Lyophilized protein should be stored at -20˚C, though stable at room temperature for 3 weeks. STORAGE CONDITIONS: Reconstituted protein solution can be stored at 4-7˚C for 2-7 days. Aliquots of reconstituted samples are stable at -20˚C for 3 months. Additional Info OFFICIAL SYMBOL: ABCB5 ALTERNATE NAMES: ATP-binding cassette sub-family B member 5, P-glycoprotein ABCB5, ABCB5 P-gp, ABCB5 ACCESSION NO.: Q2M3G0 GENE ID: 340273 Background and References ATP-binding cassette sub-family B member 5(ABCB5) is a plasma membrane-spanning protein. ABCB5 is principally expressed in physiological skin and human malignant melanoma. ABCB5 has been suggested to regulate skin progenitor cell fusion and mediate chemotherapeutic drug resistance in stem-like tumor cell subpopulations in BACKGROUND: human malignant melanoma. It is commonly over-expressed on circulating melanoma tumour cells. -

Functional Studies on the MRP1 Multidrug Transporter: Characterization of ABC-Signature Mutant Variants
ANTICANCER RESEARCH 24: 449-456 (2004) Functional Studies on the MRP1 Multidrug Transporter: Characterization of ABC-signature Mutant Variants ZS. SZENTPÉTERY1,2, B. SARKADI1, É. BAKOS2 and A. VÁRADI2 1National Medical Center, Institute of Haematology and Immunology, Membrane Research Group of the Hungarian Academy of Sciences, Budapest; 2Institute of Enzymology, Biological Research Center, Hungarian Academy of Sciences, Budapest, Hungary Abstract. Background: MRP1 is a key multidrug resistance these agents below the cell-killing threshold, thus conferring ATP-binding Cassette (ABC) transporter in tumor cells. A resistance to many structurally dissimilar anticancer drugs. functionally important signature motif is conserved within all One of the proteins causing this phenotype is the human ABC domains. Our current studies aimed to elucidate the role Multidrug Resistance Protein (MRP1), which confers of these motifs in the cooperation of MRP1 ABC domains. resistance to a wide variety of anticancer drugs (1), but has Materials and Methods: We designed human MRP1 mutants also been shown to be a high affinity primary active based on a bacterial ABC structure. Conserved leucines (Leu) transporter for glutathione (GS)-conjugates (e.g. LTC4 – see were replaced by arginines (Arg), while glycines (Gly) were 2, 3). MRP1 transports large hydrophobic drugs, playing an substituted for aspartic acids (Asp). The activity of these important role in the chemotherapy resistance of several mutants was assayed by measuring ATPase activity and types of cancer cells, and cellular GS seem to be an vesicular transport. ATP-binding and transition-state formation important modulator in these transport functions (2, 4-9). were studied by a photoreactive ATP analog. -

Inhibiting ABCG2 with Sorafenib
Published OnlineFirst May 16, 2012; DOI: 10.1158/1535-7163.MCT-12-0215 Molecular Cancer Therapeutic Discovery Therapeutics New Use for an Old Drug: Inhibiting ABCG2 with Sorafenib Yinxiang Wei1,3, Yuanfang Ma3, Qing Zhao1,4, Zhiguang Ren1,3, Yan Li1, Tingjun Hou2, and Hui Peng1 Abstract Human ABCG2, a member of the ATP-binding cassette transporter superfamily, represents a promising target for sensitizing MDR in cancer chemotherapy. Although lots of ABCG2 inhibitors were identified, none of them has been tested clinically, maybe because of several problems such as toxicity or safety and pharma- cokinetic uncertainty of compounds with novel chemical structures. One efficient solution is to rediscover new uses for existing drugs with known pharmacokinetics and safety profiles. Here, we found the new use for sorafenib, which has a dual-mode action by inducing ABCG2 degradation in lysosome in addition to inhibiting its function. Previously, we reported some novel dual-acting ABCG2 inhibitors that showed closer similarity to degradation-induced mechanism of action. On the basis of these ABCG2 inhibitors with diverse chemical structures, we developed a pharmacophore model for identifying the critical pharmacophore features necessary for dual-acting ABCG2 inhibitors. Sorafenib forms impressive alignment with the pharmacophore hypothesis, supporting the argument that sorafenib is a potential ABCG2 inhibitor. This is the first article that sorafenib may be a good candidate for chemosensitizing agent targeting ABCG2-mediated MDR. This study may facilitate the rediscovery of new functions of structurally diverse old drugs and provide a more effective and safe way of sensitizing MDR in cancer chemotherapy. Mol Cancer Ther; 11(8); 1693–702. -

Coexpression of ATP-Binding Cassette Proteins ABCG5 and ABCG8 Permits Their Transport to the Apical Surface
Coexpression of ATP-binding cassette proteins ABCG5 and ABCG8 permits their transport to the apical surface Gregory A. Graf, … , Jonathan C. Cohen, Helen H. Hobbs J Clin Invest. 2002;110(5):659-669. https://doi.org/10.1172/JCI16000. Article Cardiology Mutations in either ATP-binding cassette (ABC) G5 or ABCG8 cause sitosterolemia, an autosomal recessive disorder of sterol trafficking. To determine the site of action of ABCG5 and ABCG8, we expressed recombinant, epitope-tagged mouse ABCG5 and ABCG8 in cultured cells. Both ABCG5 and ABCG8 underwent N-linked glycosylation. When either protein was expressed individually in cells, the N-linked sugars remained sensitive to Endoglycosidase H (Endo H). When ABCG5 and ABCG8 were coexpressed, the attached sugars were Endo H–resistant and neuraminidase-sensitive, indicating that the proteins were transported to the trans-Golgi complex. The mature, glycosylated forms of ABCG5 and ABCG8 coimmunoprecipitated, consistent with heterodimerization of these two proteins. The Endo H–sensitive forms of ABCG5 and ABCG8 were confined to the endoplasmic reticulum (ER), whereas the mature forms were present in non-ER fractions in cultured hepatocytes. Immunoelectron microscopy revealed ABCG5 and ABCG8 on the plasma membrane of these cells. In polarized WIF-B cells, recombinant ABCG5 localized to the apical (canalicular) membrane when coexpressed with ABCG8, but not when expressed alone. To our knowledge this is the first direct demonstration that trafficking of an ABC half-transporter to the cell surface requires the presence of its dimerization partner. Find the latest version: https://jci.me/16000/pdf Coexpression of ATP-binding cassette See the related Commentary beginning on page 605. -

The Putative Mitochondrial Protein ABCB6
Shifting the Paradigm: The Putative Mitochondrial Protein ABCB6 Resides in the Lysosomes of Cells and in the Plasma Membrane of Erythrocytes Katalin Kiss, Anna Brozik, Nora Kucsma, Alexandra Toth, Melinda Gera, Laurence Berry, Alice Vallentin, Henri Vial, Michel Vidal, Gergely Szakacs To cite this version: Katalin Kiss, Anna Brozik, Nora Kucsma, Alexandra Toth, Melinda Gera, et al.. Shifting the Paradigm: The Putative Mitochondrial Protein ABCB6 Resides in the Lysosomes of Cells and in the Plasma Membrane of Erythrocytes. PLoS ONE, Public Library of Science, 2012, 7 (5), pp.e37378. 10.1371/journal.pone.0037378. hal-02309092 HAL Id: hal-02309092 https://hal.archives-ouvertes.fr/hal-02309092 Submitted on 25 May 2021 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Distributed under a Creative Commons Attribution| 4.0 International License Shifting the Paradigm: The Putative Mitochondrial Protein ABCB6 Resides in the Lysosomes of Cells and in the Plasma Membrane of Erythrocytes Katalin Kiss1, Anna Brozik1, Nora Kucsma1, Alexandra Toth1, Melinda Gera1, -

Cardiovascular Disease Products
Cardiovascular Disease Products For more information, visit: www.bosterbio.com Cardiovascular Disease Research Cardiovascular disease is the leading cause of death in developed nations. Boster Bio aims to supply researchers with high-quality antibodies and ELISA kits so they can make new discoveries and help save lives. In this catalogue you will find a comprehensive list of high-affinity Boster antibodies and high sensitivity Boster ELISA kits targeted at proteins associated with cardiovascular disease. Boster: The Fastest Growing About Bosterbio Antibody Company In 2015 Boster is an antibody manufacturer founded in 1993 by histologist Steven Xia. Over the past two decades, Boster and its products have been cited in over 20,000 publications and counting. The firm specializes in developing antibodies and ELISA kits that feature high affinity, Boster Bio received the CitaAb award for high specificity at affordable the greatest increase in number of prices. citations during 2015 than any other antibody manufacturer. Table of Contents Boster Cardiovascular Disease Related Antibodies…………..………..... 2 Boster Cardiovascular Disease Related ELISA Kits……………………..…. 9 1 High Affinity Boster Antibodies Boster supplies only the highest quality antibodies. Our high-affinity polyclonal and monoclonal antibodies are thoroughly validated by Western Blotting, Immunohistochemistry and ELISA. This is our comprehensive catalog of our antibody products related to cardiovascular disease, sorted in alphabetical order by target gene name. Catalog No Product Name -
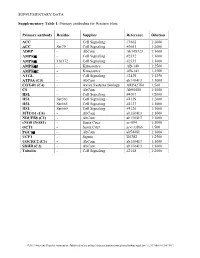
Supplementary Table 1
SUPPLEMENTARY DATA Supplementary Table 1. Primary antibodies for Western blots. Primary antibody Residue Supplier Reference Dilution ACC - Cell Signaling #3662 1:2000 ACC Ser79 Cell Signaling #3661 1:2000 ADRP - AbCam Ab108323 1:1000 AMPKα - Cell Signaling #2532 1:1000 AMPKα Thr172 Cell Signaling #2535 1:1000 AMPKα1 - Kinasource AB-140 1:2500 AMPKα2 - Kinasource AB-141 1:2500 ATGL - Cell Signaling #2439 1:1250 ATP5A (C5) - AbCam ab110413 1:1000 COX4l1 (C4) - Aviva Systems Biology ARP42784 1:500 CS - AbCam Ab96600 1:1000 HSL - Cell Signaling #4107 1:2000 HSL Ser563 Cell Signaling #4139 1:2000 HSL Ser565 Cell Signaling #4137 1:1000 HSL Ser660 Cell Signaling #4126 1:1000 MTCO1 (C4) - AbCam ab110413 1:1000 NDUFB8 (C1) - AbCam ab110413 1:1000 eNOS (NOS3) - Santa Cruz sc-654 1:1000 OCT1 - Santa Cruz sc-133866 1:500 PGC1α - AbCam ab54481 1:1000 UCP1 - Sigma U6382 1:2500 UQCRC2 (C3) - AbCam ab110413 1:1000 SDHB (C2) - AbCam ab110413 1:1000 Tubulin - Cell Signaling #2148 1:2000 ©2013 American Diabetes Association. Published online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db13-0194/-/DC1 SUPPLEMENTARY DATA Supplementary Table 2. Primer sequences for qRT-PCR. Gene Accession number Forward primer Reverse primer Abca1 NM_013454.3 CCCAGAGCAAAAAGCGACTC GGTCATCATCACTTTGGTCCTTG Abcg5 NM_031884 TGTCCTACAGCGTCAGCAACC GGCCACTCTCGATGTACAAGG Abcg8 NM_026180 TCCTGTGAGCTGGGCATCCGA CCCGCAGCCTGAGCTCCCTAT Acaca NM_133360.2 CAGCTGGTGCAGAGGTACCG TCTACTCGCAGGTACTGCCG Acacb NM_133904.2 GCGCCTACTATGAGGCCCAGCA ACAAACTCGGCTGGGGACGC Acly NM_134037.2 -

Overexpression of ABCG5 and ABCG8 Promotes Biliary Cholesterol Secretion and Reduces Fractional Absorption of Dietary Cholestero
Overexpression of ABCG5 See the related Commentary beginning on page 605. and ABCG8 promotes biliary cholesterol secretion and reduces fractional absorption of dietary cholesterol Liqing Yu,1 Jia Li-Hawkins,1 Robert E. Hammer,2,3 Knut E. Berge,1 Jay D. Horton,1 Jonathan C. Cohen,4 and Helen H. Hobbs1 1Department of Molecular Genetics and McDermott Center for Human Growth and Development, 2Department of Biochemistry, 3Howard Hughes Medical Institute, and 4Center for Human Nutrition, University of Texas Southwestern Medical Center at Dallas, Dallas, Texas, USA Two ATP-binding cassette (ABC) transporters, ABCG5 and ABCG8, have been proposed to limit sterol absorption and to promote biliary sterol excretion in humans. To test this hypothesis, a P1 clone containing the human ABCG5 and ABCG8 genes was used to generate transgenic mice. The transgenes were expressed primarily in the liver and small intestine, mirroring the expression pattern of the endogenous genes. Transgene expression only modestly affected plasma and liver cholesterol levels but profoundly altered cholesterol transport. The fractional absorption of dietary cholesterol was reduced by about 50%, and biliary cholesterol levels were increased more than fivefold. Fecal neu- tral sterol excretion was increased three- to sixfold and hepatic cholesterol synthesis increased two- to fourfold in the transgenic mice. No significant changes in the pool size, composition, and fecal excretion of bile acids were observed in the transgenic mice. Transgene expression attenuated the increase in hepatic cholesterol content induced by consumption of a high cholesterol diet. These results demonstrate that increased expression of ABCG5 and ABCG8 selectively drives biliary neutral sterol secretion and reduces intestinal cholesterol absorption, leading to a selective increase in neu- tral sterol excretion and a compensatory increase in cholesterol synthesis. -

A Case of Ezetimibe-Effective Hypercholesterolemia with a Novel Heterozygous Variant in ABCG5
2020, 67 (11), 1099-1105 Original A case of ezetimibe-effective hypercholesterolemia with a novel heterozygous variant in ABCG5 Yujiro Nakano1), Chikara Komiya1), Hitomi Shimizu2), 3), Hiroyuki Mishima3), Kumiko Shiba1), Kazutaka Tsujimoto1), Kenji Ikeda1), Kenichi Kashimada4), Sumito Dateki2), Koh-ichiro Yoshiura3), Yoshihiro Ogawa5) and Tetsuya Yamada1) 1) Department of Molecular Endocrinology and Metabolism, Graduate School of Medical and Dental Sciences, Tokyo Medical and Dental University, Tokyo 113-8519, Japan 2) Department of Pediatrics, Graduate School of Biomedical Sciences, Nagasaki University, Nagasaski 852-8501, Japan 3) Department of Human Genetics, Graduate School of Biomedical Sciences, Nagasaki University, Nagasaki 852-8501, Japan 4) Department of Pediatrics and Developmental Biology, Graduate School of Medical and Dental Sciences, Tokyo Medical and Dental University, Tokyo 113-8519, Japan 5) Department of Medicine and Bioregulatory Science, Graduate School of Medical Sciences, Kyushu University, Fukuoka 812-8582, Japan Abstract. Sitosterolemia is caused by homozygous or compound heterozygous gene mutations in either ATP-binding cassette subfamily G member 5 (ABCG5) or 8 (ABCG8). Since ABCG5 and ABCG8 play pivotal roles in the excretion of neutral sterols into feces and bile, patients with sitosterolemia present elevated levels of serum plant sterols and in some cases also hypercholesterolemia. A 48-year-old woman was referred to our hospital for hypercholesterolemia. She had been misdiagnosed with familial hypercholesterolemia at the age of 20 and her serum low-density lipoprotein cholesterol (LDL-C) levels had remained about 200–300 mg/dL at the former clinic. Although the treatment of hydroxymethylglutaryl-CoA (HMG-CoA) reductase inhibitors was ineffective, her serum LDL-C levels were normalized by ezetimibe, a cholesterol transporter inhibitor. -

Identification of Novel Rare ABCC1 Transporter Mutations in Tumor
cells Article Identification of Novel Rare ABCC1 Transporter Mutations in Tumor Biopsies of Cancer Patients Onat Kadioglu 1, Mohamed Saeed 1, Markus Munder 2, Andreas Spuller 3, Henry Johannes Greten 4,5 and Thomas Efferth 1,* 1 Department of Pharmaceutical Biology, Institute of Pharmacy and Biochemistry, Johannes Gutenberg University, 55128 Mainz, Germany; [email protected] (O.K.); [email protected] (M.S.) 2 Third Department of Medicine (Hematology, Oncology, and Pneumology), University Medical Center of the Johannes Gutenberg University Mainz, 55131 Mainz, Germany; [email protected] 3 Clinic for Gynecology and Obstetrics, 76131 Karlsruhe, Germany; [email protected] 4 Abel Salazar Biomedical Sciences Institute, University of Porto, 4099-030 Porto, Portugal; [email protected] 5 Heidelberg School of Chinese Medicine, 69126 Heidelberg, Germany * Correspondence: eff[email protected]; Tel.: +49-6131-392-5751; Fax: 49-6131-392-3752 Received: 30 December 2019; Accepted: 23 January 2020; Published: 26 January 2020 Abstract: The efficiency of chemotherapy drugs can be affected by ATP-binding cassette (ABC) transporter expression or by their mutation status. Multidrug resistance is linked with ABC transporter overexpression. In the present study, we performed rare mutation analyses for 12 ABC transporters related to drug resistance (ABCA2, -A3, -B1, -B2, -B5, -C1, -C2, -C3, -C4, -C5, -C6, -G2) in a dataset of 18 cancer patients. We focused on rare mutations resembling tumor heterogeneity of ABC transporters in small tumor subpopulations. Novel rare mutations were found in ABCC1, but not in the other ABC transporters investigated. Diverse ABCC1 mutations were found, including nonsense mutations causing premature stop codons, and compared with the wild-type protein in terms of their protein structure. -

Common Genetic Variations Involved in the Inter-Individual Variability Of
nutrients Review Common Genetic Variations Involved in the Inter-Individual Variability of Circulating Cholesterol Concentrations in Response to Diets: A Narrative Review of Recent Evidence Mohammad M. H. Abdullah 1 , Itzel Vazquez-Vidal 2, David J. Baer 3, James D. House 4 , Peter J. H. Jones 5 and Charles Desmarchelier 6,* 1 Department of Food Science and Nutrition, Kuwait University, Kuwait City 10002, Kuwait; [email protected] 2 Richardson Centre for Functional Foods & Nutraceuticals, University of Manitoba, Winnipeg, MB R3T 6C5, Canada; [email protected] 3 United States Department of Agriculture, Agricultural Research Service, Beltsville, MD 20705, USA; [email protected] 4 Department of Food and Human Nutritional Sciences, University of Manitoba, Winnipeg, MB R3T 2N2, Canada; [email protected] 5 Nutritional Fundamentals for Health, Vaudreuil-Dorion, QC J7V 5V5, Canada; [email protected] 6 Aix Marseille University, INRAE, INSERM, C2VN, 13005 Marseille, France * Correspondence: [email protected] Abstract: The number of nutrigenetic studies dedicated to the identification of single nucleotide Citation: Abdullah, M.M.H.; polymorphisms (SNPs) modulating blood lipid profiles in response to dietary interventions has Vazquez-Vidal, I.; Baer, D.J.; House, increased considerably over the last decade. However, the robustness of the evidence-based sci- J.D.; Jones, P.J.H.; Desmarchelier, C. ence supporting the area remains to be evaluated. The objective of this review was to present Common Genetic Variations Involved recent findings concerning the effects of interactions between SNPs in genes involved in cholesterol in the Inter-Individual Variability of metabolism and transport, and dietary intakes or interventions on circulating cholesterol concen- Circulating Cholesterol trations, which are causally involved in cardiovascular diseases and established biomarkers of Concentrations in Response to Diets: cardiovascular health.