An Entamoeba-Specific Mitosomal Membrane Protein with Potential Association to the Golgi Apparatus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Protein Sequencing Production of Ions for Mass Spectrometry
Lecture 1 Introduction- Protein Sequencing Production of Ions for Mass Spectrometry Nancy Allbritton, M.D., Ph.D. Department of Physiology & Biophysics 824-9137 (office) [email protected] Office- Rm D349 Medical Science D Bldg. Introduction to Proteins Amino Acid- structural unit of a protein α carbon Amino acids- linked by peptide (amide) bond Amino Acids Proteins- 20 amino acids (Recall DNA- 4 bases) R groups- Varying size, shape, charge, H-bonding capacity, & chemical reactivity Introduction to Proteins Polypeptide Chain (Protein) - Many amino acids linked by peptide bonds By convention: Residue 1 starts at amino terminus. Polypeptides- a. Main chain i.e. regularly repeating portion b. Side chains- variable portion Introduction to Proteins 25,000 human genes >2X106 proteins Natural Proteins - Typically 50-2000 amino acids i.e. 550-220,000 molecular weight Over 200 different types of post-translational modifications. Ex: proteolysis, phosphorylation, acetylation, glycosylation S S S S S S Ex: Insulin Protein Complexity Is Very Large Over 200 different types of post-translational modifications. The Problem of Protein Sequencing. Edman Degradation: Step-wise cleavage of an amino acid from the amino terminus of a peptide. Alanine Gly 1 2 Reacts with uncharged NH3 1 2 Gly 1 2 Cyclizes & Releases in Mild Acid H N-Gly 2 2 2 PTH-Alanine Edman Degradation 1. Must be short peptide (<50 a.a.) amino acid release- 98% efficiency proteins- must fragment (CNBr or trypsin) Trypsin 2. Frequently fails due to a blocked amino terminus 3. Intolerant of impurities 4. Tedious & time consuming (hours-days) 1 amino acid cycle- 2 hours Solution: Mass Spectrometry 1. -
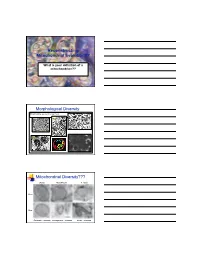
Reconstructing Mitochondrial Evolution?? Morphological Diversity Mitochondrial Diversity???
Reconstructing Mitochondrial Evolution?? What is your definition of a mitochondrion?? Morphological Diversity Mitochondria as we all know them: Suprarenal gland Liver cell Plasma cell Adrenal cortex Mitochondrial Diversity??? Chicken Neocallimastix T. foetus 100 nm 50 nm Entamoeba - mitosome microsporidian - mitosome Giardia - mitosome Reconstructing Evolution Mitochondrial evolution well established endosymbiotic theory α-proteobacterium - Rickettsia prowazekii Hydrogenosomal evolution No DNA NOW 2 examples Nyctotherus and Blastocystis (MLO) Several proteins similar to mitochondria Mitosome evolution No DNA Few proteins identified similar to mitochondria Origins via Endosymbiosis Current dogma - mitochondria and related Aerobic α-proteobacterium prokaryote gave rise to present day mitochondria. organelles arose just once in evolution Are hydrogenosomes and mitosomes of anaerobic protists derived from the same proto-mitochondrion? Evidence for: accumulating evidence for several proteins that are currently found in mitochondria - Proteins of Fe-S cluster formation. Scenario A Common ancestor facultative Scenario B organism? degenerate mitochondrion invoke lateral gene transfer from anaerobic prokaryotes Hypotheses for Mito Acquisition Mitochondrion-related Organelles 2 way to interpret this summary - can you think of both? Groups with mitochondrial homologues Hjort et al Phil. Trans. R. Soc. B 2010 365, 713-727 Origin of Hydrogenosomes (Mitosomes?) 1. a)Conversion of Mitochondria HYD b)Common ancestor with mitochondria ? HYD 2. Independent Origin α-proteobacterium ? - anaerobic bacterium HYD Organelles - origins and biogenesis Approaches: (1) Conduct phylogenetic analyses of similar proteins Hsp70 Fd Hsp60 Isc subunits (2) Examine protein targeting to the organelle matrix protein targeting membrane protein targeting (3) Characterize membrane/translocation components These components could have evolved as the endosymbiont was converted to organelle. Reveals evolutionary history. -

9 3 Innovirolgy Examples and Applications
9.3: Examples and applications 1) Now that you have an understanding of the mechanisms behind the phage display system, let’s have a look at how it’s used in research. i) Phage display has a broad spectrum of applications. This can go from improvement of enzymes, to proteome analysis or drug and target discovery but is certainly not restricted to these. 2) The first thing I want to focus on is the optimization of enzymes. A large variety of enzymes are being used today in different fields of industry and biotech. These enzymes are originally made by organisms for a specific function in a specific niche or environment, like inside the cell cytoplasm. Therefore, they often have undesirable properties for the function or environment we want to use them in. They might not work fast enough, be unstable in the conditions they are used in or do not work on the desired substrate. Phage display can aid in improving these enzymes by selecting for example for better thermostability, activity or enhanced substrate binding. i) Let’s start with stability. Take for example an enzyme that is normally present In a cell at cytoplasm at 37°C. However when we use it in an industrial process where we need 50°C, the enzyme becomes unstable and denatures. In phage display we can make a library of mutagenized enzyme and display them on, in this case, the gene 3 protein of the phage. In the binding step of the biopanning most phages will not be able to bind the enzyme substrate at 50°C as they are denatured. -

Single-Molecule Peptide Fingerprinting
Single-molecule peptide fingerprinting Jetty van Ginkela,b, Mike Filiusa,b, Malwina Szczepaniaka,b, Pawel Tulinskia,b, Anne S. Meyera,b,1, and Chirlmin Joo (주철민)a,b,1 aKavli Institute of Nanoscience, Delft University of Technology, 2629HZ Delft, The Netherlands; and bDepartment of Bionanoscience, Delft University of Technology, 2629HZ Delft, The Netherlands Edited by Alan R. Fersht, Gonville and Caius College, Cambridge, United Kingdom, and approved February 21, 2018 (received for review May 1, 2017) Proteomic analyses provide essential information on molecular To obtain ordered determination of fluorescently labeled amino pathways of cellular systems and the state of a living organism. acids, we needed a molecular probe that can scan a peptide in a Mass spectrometry is currently the first choice for proteomic processive manner. We adopted a naturally existing molecular analysis. However, the requirement for a large amount of sample machinery, the AAA+ protease ClpXP from Escherichia coli.The renders a small-scale proteomics study challenging. Here, we ClpXP protein complex is an enzymatic motor that unfolds and demonstrate a proof of concept of single-molecule FRET-based degrades protein substrates. ClpX monomers form a homohexa- + protein fingerprinting. We harnessed the AAA protease ClpXP to meric ring (ClpX6) that can exercise a large mechanical force to scan peptides. By using donor fluorophore-labeled ClpP, we se- unfold proteins using ATP hydrolysis (11, 12). Through iterative quentially read out FRET signals from acceptor-labeled amino acids rounds of force-generating power strokes, ClpX6 translocates of peptides. The repurposed ClpXP exhibits unidirectional process- substrates through the center of its ring in a processive manner ing with high processivity and has the potential to detect low- (13, 14), with extensive promiscuity toward unnatural substrate abundance proteins. -

Intrinsic Indicators for Specimen Degradation
Laboratory Investigation (2013) 93, 242–253 & 2013 USCAP, Inc All rights reserved 0023-6837/13 $32.00 Intrinsic indicators for specimen degradation Jie Li1, Catherine Kil1, Kelly Considine1, Bartosz Smarkucki1, Michael C Stankewich1, Brian Balgley2 and Alexander O Vortmeyer1 Variable degrees of molecular degradation occur in human surgical specimens before clinical examination and severely affect analytical results. We therefore initiated an investigation to identify protein markers for tissue degradation assessment. We exposed 4 cell lines and 64 surgical/autopsy specimens to defined periods of time at room temperature before procurement (experimental cold ischemic time (CIT)-dependent tissue degradation model). Using two-dimen- sional fluorescence difference gel electrophoresis in conjunction with mass spectrometry, we performed comparative proteomic analyses on cells at different CIT exposures and identified proteins with CIT-dependent changes. The results were validated by testing clinical specimens with western blot analysis. We identified 26 proteins that underwent dynamic changes (characterized by continuous quantitative changes, isoelectric changes, and/or proteolytic cleavages) in our degradation model. These changes are strongly associated with the length of CIT. We demonstrate these proteins to represent universal tissue degradation indicators (TDIs) in clinical specimens. We also devised and implemented a unique degradation measure by calculating the quantitative ratio between TDIs’ intact forms and their respective degradation- -
![M.Sc. [Botany] 346 13](https://docslib.b-cdn.net/cover/3507/m-sc-botany-346-13-923507.webp)
M.Sc. [Botany] 346 13
cover page as mentioned below: below: mentioned Youas arepage instructedcover the to updateupdate to the coverinstructed pageare asYou mentioned below: Increase the font size of the Course Name. Name. 1. IncreaseCourse the theof fontsize sizefont ofthe the CourseIncrease 1. Name. use the following as a header in the Cover Page. Page. Cover 2. the usein the followingheader a as as a headerfollowing the inuse the 2. Cover Page. ALAGAPPAUNIVERSITY UNIVERSITYALAGAPPA [Accredited with ’A+’ Grade by NAAC (CGPA:3.64) in the Third Cycle Cycle Third the in (CGPA:3.64) [AccreditedNAAC by withGrade ’A+’’A+’ Gradewith by NAAC[Accredited (CGPA:3.64) in the Third Cycle and Graded as Category–I University by MHRD-UGC] MHRD-UGC] by University and Category–I Graded as as Graded Category–I and University by MHRD-UGC] M.Sc. [Botany] 003 630 – KARAIKUDIKARAIKUDI – 630 003 346 13 EDUCATION DIRECTORATEDISTANCE OF OF DISTANCEDIRECTORATE EDUCATION BIOLOGICAL TECHNIQUES IN BOTANY I - Semester BOTANY IN TECHNIQUES BIOLOGICAL M.Sc. [Botany] 346 13 cover page as mentioned below: below: mentioned Youas arepage instructedcover the to updateupdate to the coverinstructed pageare asYou mentioned below: Increase the font size of the Course Name. Name. 1. IncreaseCourse the theof fontsize sizefont ofthe the CourseIncrease 1. Name. use the following as a header in the Cover Page. Page. Cover 2. the usein the followingheader a as as a headerfollowing the inuse the 2. Cover Page. ALAGAPPAUNIVERSITY UNIVERSITYALAGAPPA [Accredited with ’A+’ Grade by NAAC (CGPA:3.64) in the Third Cycle Cycle Third the in (CGPA:3.64) [AccreditedNAAC by withGrade ’A+’’A+’ Gradewith by NAAC[Accredited (CGPA:3.64) in the Third Cycle and Graded as Category–I University by MHRD-UGC] MHRD-UGC] by University and Category–I Graded as as Graded Category–I and University by MHRD-UGC] M.Sc. -

Electrophoresis
B2144 Cover.qxd 9/1/98 8:10 AM Page 2 Ready Gel System RESOURCE GUIDE Bio-Rad Laboratories Life Science Australia, Bio-Rad Laboratories Pty Limited, Block Y Unit 1, Regents Park Industrial Estate, 391 Park Road, Regents Park, NSW 2143 • Group Phone 02-9914-2800 • Fax 02-9914-2889 Austria, Bio-Rad Laboratories Ges.m.b.H., Auhofstrasse 78D, A-1130 Wien • Phone (1) 877 89 01 • Fax (1) 876 56 29 Belgium, Bio-Rad Laboratories S.A.-N.V., Begoniastraat 5, B-9810 Nazareth EKE • Phone 09-385 55 11 • Fax 09-385 65 54 2000 Alfred Nobel Drive Canada, Bio-Rad Laboratories (Canada) Ltd., 5671 McAdam Road, Mississauga, Ontario L4Z 1N9 • Phone (905) 712-2771 • Fax (905) 712-2990 Hercules, California 94547 China, Bio-Rad China (Beijing), 14 Zhi Chun Road, Hai Dian District, Beijing 100 008 • Phone 86-10-62051850 • Fax 86-10-62051876 Telephone (510) 741-1000 Denmark, Bio-Rad Laboratories, Symbion Science Park, Fruebjergvej 3, DK-2100 København Ø • Phone 39 17 9947 • Fax 39 27 1698 Fax: (510) 741-5800 Finland, Bio-Rad Laboratories, Pihatörmä 1A 02240, Espoo, • Phone 90 804 2200 • Fax 90 804 1100 www.bio-rad.com France, Bio-Rad S.A., 94/96 rue Victor Hugo, B.P. 220, 94 203 Ivry Sur Seine Cedex • Phone (1) 43 90 46 90 • Fax (1) 46 71 24 67 Germany, Bio-Rad Laboratories GmbH, Heidemannstraße 164, D-80939 München • Phone 089 31884-0 • Fax 089 31884-100 Hong Kong, Bio-Rad Pacific Ltd., Unit 1111, 11/F., New Kowloon Plaza, 38 Tai Kok Tsui Road, Tai Kok Tsui, Kowloon, Hong Kong • Phone 852-2789-3300 • Fax 852-2789-1257 India, Bio-Rad Laboratories, India (Pvt.) Ltd., C-248 Defence Colony, New Delhi 110 024 • Phone 91-11-461-0103 • Fax 91-11-461-0765 Israel, Bio-Rad Laboratories Ltd., 12 Homa Street, P.O. -

Mitosomes in Entamoeba Histolytica Contain a Sulfate Activation Pathway
Mitosomes in Entamoeba histolytica contain a sulfate activation pathway Fumika Mi-ichi, Mohammad Abu Yousuf, Kumiko Nakada-Tsukui, and Tomoyoshi Nozaki1 Department of Parasitology, National Institute of Infectious Diseases, Tokyo 162-8640, Japan Edited by Andrew Roger, Dalhousie University, Halifax, NS, Canada, and accepted by the Editorial Board October 19, 2009 (received for review June 25, 2009) Hydrogenosomes and mitosomes are mitochondrion-related or- and M. balamuthi lack the ISC system, and instead possess the ganelles in anaerobic/microaerophilic eukaryotes with highly re- nitrogen fixation (NIF) system, which is most likely derived from an duced and divergent functions. The full diversity of their content ancestral nitrogen fixing -proteobacterium by lateral gene transfer and function, however, has not been fully determined. To under- (22, 24). Only 5 proteins have been demonstrated in E. histolytica stand the central role of mitosomes in Entamoeba histolytica,a mitosomes: Cpn60 (8–10, 12), Cpn10 (13), mitochondrial Hsp70 parasitic protozoon that causes amoebic dysentery and liver ab- (11, 15), pyridine nucleotide transhydrogenase (PNT) (2, 8), and scesses, we examined the proteomic profile of purified mitosomes. mitochondria carrier family (MCF, ADP/ATP transporter) (14), Using 2 discontinuous Percoll gradient centrifugation and MS and the central role of mitosomes in E. histolytica remains unknown. analysis, we identified 95 putative mitosomal proteins. Immuno- Analysis of the genome of E. histolytica has not revealed any fluorescence assay showed that 3 proteins involved in sulfate additional information regarding the function of mitosomes and activation, ATP sulfurylase, APS kinase, and inorganic pyrophos- thus, a proteomic analysis of mitosomes seems to be the best phatase, as well as sodium/sulfate symporter, involved in sulfate approach to understand its structure and function (1, 2). -

A Novel ADP/ATP Transporter in the Mitosome of the Microaerophilic Human Parasite Entamoeba Histolytica
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Current Biology, Vol. 15, 737–742, April 26, 2005, ©2005 Elsevier Ltd All rights reserved. DOI 10.1016/j.cub.2005.02.068 A Novel ADP/ATP Transporter in the Mitosome of the Microaerophilic Human Parasite Entamoeba histolytica Ka Wai Chan,1,5 Dirk-Jan Slotboom,1,5 Sian Cox,3 ses confirm that the Entamoeba ADP/ATP carrier is T. Martin Embley,2,* Olivier Fabre,1 distinct from archetypal mitochondrial ADP/ATP carri- Mark van der Giezen,3,6 Marilyn Harding,1 ers, an observation that is supported by its different David S. Horner,4 Edmund R.S. Kunji,1,* substrate and inhibitor specificity. Because many Gloria León-Avila,3,7 and Jorge Tovar3 functions of yeast and human mitochondria rely on 1Dunn Human Nutrition Unit solutes transported by specialized members of this Medical Research Council family, the Entamoeba mitosome must contain only Hills Road a small subset of these processes requiring adenine Cambridge CB2 2XY nucleotide exchange. United Kingdom 2 School of Biology The Devonshire Building Results and Discussion University of Newcastle upon Tyne Newcastle NE1 7RU Identification of an MCF Homolog United Kingdom on the Entamoeba histolytica Genome 3 School of Biological Sciences We identified a homolog of the mitochondrial carrier Royal Holloway family (MCF) on the Entamoeba histolytica genome (TIGR) University of London with BLAST searches. The translated protein contains a Egham, Surrey TW20 0EX tripartite structure [8], signature motifs (IPR001993), and United Kingdom other features typical of MCF members (Figure 1A). -

De Novo Peptide Sequencing Tutorial
http://ionsource.com/tutorial/DeNovo/introduction.htm - De Novo Peptide Sequencing Tutorial 1 http://ionsource.com/tutorial/DeNovo/introduction.htm Table of Contents Introduction - Peptide Fragmentation Nomenclature - b and y Ions - The Rules (16 simple rules) - The Protocol - Example MS/MS Spectrum - • low mass • mid mass • high mass - First De Novo Exercise (Ion Trap Data) - • Answer Page - Second De Novo Exercise (q-TOF Data) - • Answer page • Tutorial References and Additional Suggested Reading - • De Novo Tables - AA Residue Mass, Mass Conflict, Di-peptide Mass Residue Combinations 2 http://ionsource.com/tutorial/DeNovo/introduction.htm 3 http://ionsource.com/tutorial/DeNovo/introduction.htm Introduction De novo is Latin for, "over again", or "anew". A popular definition for "de novo peptide sequen- cing" is, peptide sequencing performed without prior knowledge of the amino acid sequence. Usually this rule is imposed by Edman degradation practitioners who perform de novo sequencing day in and day out, and perhaps feel a little bit threatened by that half million dollar mass spectrometer sitting down the hall, that can supposedly sequence peptides in a matter of seconds, and not days. Actually, any research project should be started with as much information as pos- sible, there should never be a need to restrict your starting knowledge, unless of course you are performing a clinical trial or some other highly controlled experiment. Mass spectrometers do have the advantage when it comes to generating sequence data for peptides in low femtomole quantities. However, Edman degradation will always enjoy the advantage when pmol quantities of a peptide are available. At higher pmol quantities (2-10 pmol), Edman will often provide the exact amino acid sequence without ambiguity for a limited run of amino acids, 6-30 amino acids, usually taking 30-50 min per cycle of the sequencer. -

UNIVERSITY of ALBERTA High Sensitivity Protein Sequencing
UNIVERSITY OF ALBERTA High Sensitivity Protein Sequencing using Fluorescein Isothiocyanate for Denvatization on a Miniaturized Sequencer using Capillary EIectrophoresis with Laser hduced Fluorescence Detection for Prduct Identification. A thesis submitted to the Faculty of Graduate Studies and Research in partial Mfiment of the requirernents for the degree of Doctor of Philosophy. DEPARTMENT OF CHEMISTRY EDMONTON, ALBERTA, CANADA SPRING 2000 National Library Bibliothèque nationale 141 ,cm,, du Canada Acquisitions and Acquisitions et Bibtiographic Services services bibliographiques 395 Wellington Street 395. rue Wellington Ottawa ON K1A ON4 Ottawa ON K1A ON4 Canada Canada The author has granted a non- L'auteur a accordé une Licence non exclusive licence allowing the exclusive permettant à la National Lïbrary of Canada to Bibliothèque nationale du Canada de reproduce, loan, distribute or selI reproduire, prêter, distribuer ou copies of this thesis in rnicroform, vendre des copies de cette thèse sous paper or electronic formats. la fonne de microfiche/fïlm, de reproduction sur papier ou sur format électronique. The author retauis ownership of the L'auteur conserve la propriété du copyright in this thesis. Neither the droit d'auteur qui protège cette thèse. thesis nor substantial extracts fkom it Ni la thèse ni des extraits substantiels may be printed or otherwise de celle-ci ne doivent être imprimés reproduced without the author's ou autrement reproduits sans son permission. autorisation. For my Parents Primary sequence malysis is an important step in determining the strùctr;re- function relationship for a @en protein or peptide. By far the most popular sequencing method in use today is the Edman degradation. -

Mass Spectrometry-Based De Novo Sequencing of the Anti-FLAG-M2 Antibody Using 2 Multiple Proteases and a Dual Fragmentation Scheme
bioRxiv preprint doi: https://doi.org/10.1101/2021.01.07.425675; this version posted January 12, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 1 Mass spectrometry-based de novo sequencing of the anti-FLAG-M2 antibody using 2 multiple proteases and a dual fragmentation scheme 3 4 Authors: 5 Weiwei Peng1#, Matti F. Pronker1#, Joost Snijder1* 6 7 #equal contribution 8 *corresponding author: [email protected] 9 10 Affiliation: 11 1 BioMolecular Mass SpectroMetry and ProteoMics, Bijvoet Center for BioMolecular Research 12 and Utrecht Institute of PharMaceutical Sciences, Utrecht University, Padualaan 8, 3584 CH 13 Utrecht, The Netherlands 14 15 Keywords: 16 mass spectrometry, antibody, de novo sequencing, EThcD, stepped HCD, Herceptin, FLAG tag, 17 anti-FLAG-M2. 1 bioRxiv preprint doi: https://doi.org/10.1101/2021.01.07.425675; this version posted January 12, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 18 Abstract: 19 Antibody sequence inforMation is crucial to understanding the structural basis for antigen binding 20 and enables the use of antibodies as therapeutics and research tools. Here we deMonstrate a 21 method for direct de novo sequencing of Monoclonal IgG from the purified antibody products.