Distribution and Diversity of Polyporaceae in Western India: an Overview and Addition to Mycoflora of the Gujarat State
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Survey of Fungi at the University of Wisconsin-Waukesha Field Station
University of Wisconsin Milwaukee UWM Digital Commons Field Station Bulletins UWM Field Station Spring 1993 A survey of fungi at the University of Wisconsin- Waukesha Field Station Alan D. Parker University of Wisconsin-Waukesha Follow this and additional works at: https://dc.uwm.edu/fieldstation_bulletins Part of the Forest Biology Commons, and the Zoology Commons Recommended Citation Parker, A.D. 1993 A survey of fungi at the University of Wisconsin-Waukesha Field Station. Field Station Bulletin 26(1): 1-10. This Article is brought to you for free and open access by UWM Digital Commons. It has been accepted for inclusion in Field Station Bulletins by an authorized administrator of UWM Digital Commons. For more information, please contact [email protected]. A Survey of Fungi at the University of Wisconsin-Waukesha Field Station Alan D. Parker Department of Biological Sciences University of Wisconsin-Waukesha Waukesha, Wisconsin 53188 Introduction The University of Wisconsin-Waukesha Field Station was founded in 1967 through the generous gift of a 98 acre farm by Ms. Gertrude Sherman. The facility is located approximately nine miles west of Waukesha on Highway 18, just south of the Waterville Road intersection. The site consists of rolling glacial deposits covered with old field vegetation, 20 acres of xeric oak woods, a small lake with marshlands and bog, and a cold water stream. Other communities are being estab- lished as a result of restoration work; among these are mesic prairie, oak opening, and stands of various conifers. A long-term study of higher fungi and Myxomycetes, primarily from the xeric oak woods, was started in 1978. -

Diversity of Polyporales in the Malay Peninsular and the Application of Ganoderma Australe (Fr.) Pat
DIVERSITY OF POLYPORALES IN THE MALAY PENINSULAR AND THE APPLICATION OF GANODERMA AUSTRALE (FR.) PAT. IN BIOPULPING OF EMPTY FRUIT BUNCHES OF ELAEIS GUINEENSIS MOHAMAD HASNUL BIN BOLHASSAN FACULTY OF SCIENCE UNIVERSITY OF MALAYA KUALA LUMPUR 2013 DIVERSITY OF POLYPORALES IN THE MALAY PENINSULAR AND THE APPLICATION OF GANODERMA AUSTRALE (FR.) PAT. IN BIOPULPING OF EMPTY FRUIT BUNCHES OF ELAEIS GUINEENSIS MOHAMAD HASNUL BIN BOLHASSAN THESIS SUBMITTED IN FULFILMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY INSTITUTE OF BIOLOGICAL SCIENCES FACULTY OF SCIENCE UNIVERSITY OF MALAYA KUALA LUMPUR 2013 UNIVERSITI MALAYA ORIGINAL LITERARY WORK DECLARATION Name of Candidate: MOHAMAD HASNUL BIN BOLHASSAN (I.C No: 830416-13-5439) Registration/Matric No: SHC080030 Name of Degree: DOCTOR OF PHILOSOPHY Title of Project Paper/Research Report/Disertation/Thesis (“this Work”): DIVERSITY OF POLYPORALES IN THE MALAY PENINSULAR AND THE APPLICATION OF GANODERMA AUSTRALE (FR.) PAT. IN BIOPULPING OF EMPTY FRUIT BUNCHES OF ELAEIS GUINEENSIS. Field of Study: MUSHROOM DIVERSITY AND BIOTECHNOLOGY I do solemnly and sincerely declare that: 1) I am the sole author/writer of this work; 2) This Work is original; 3) Any use of any work in which copyright exists was done by way of fair dealing and for permitted purposes and any excerpt or extract from, or reference to or reproduction of any copyright work has been disclosed expressly and sufficiently and the title of the Work and its authorship have been acknowledge in this Work; 4) I do not have any actual -

Jamur Polyporales Di Twa Suranadi Lombok Barat
Biopendix, Volume 7, Nomor 1, Desember 2020, hlm. 49-53 JAMUR POLYPORALES DI TWA SURANADI LOMBOK BARAT Meilinda Pahriana Sulastri*1, Hasan Basri2 Program Studi Biologi, Universitas Islam Al-Azhar, Mataram, NTB Coresponding author: Meilinda Pahriana Sulastri; Email: [email protected] Abstract Background: Mushrooms are a member of the kingdom of fungi, which has a high level of diversity in Indonesia. In general, fungi will grow in humid environmental conditions, especially during the rainy season on weathered wood, litter and trees. Macro fungi are fungi that form fruiting bodies and can be observed without using a microscope. Macro fungi generally consist of members of the Ascomycota and Basidomycota groups. This study aims to determine which mushrooms are included in the Polyporales order that grows in TWA Suranadi Methods: This research is descriptive exploratory by exploring and describing the macrophages found in TWA Suranadi. Sampling was carried out by the cruise method (Cruise Method). Identification is done by matching observational data in the form of morphological characteristics and environmental conditions using reference books and various scientific journals on macrofungal species. Results: The results showed that there were 7 species from 2 fungal families of the Polyporales order, namely: Trametes sp., Microporus sp. 1, Microporus sp. 2, Polyporus sp. 1, Polyporus sp. 2, Datronia sp. and Ganoderma sp. Conclusion: Based on the identification results, 2 families and 8 species were found belonging to the Polyporales order in TWA Suranadi, namely Trametes sp., Microporus sp. 1, Microporus sp. 2, Polyporus sp. 1, Polyporus sp. 2, Datronia sp., and Ganoderma sp. Keywords: fungi, Polyporales, TWA Suranadi Abstrak Latar Belakang: Jamur termasuk dalam anggota kingdom fungi yang memiliki tingkat keanekaragaman tinggi di Indonesia. -

Phylogenetic Classification of Trametes
TAXON 60 (6) • December 2011: 1567–1583 Justo & Hibbett • Phylogenetic classification of Trametes SYSTEMATICS AND PHYLOGENY Phylogenetic classification of Trametes (Basidiomycota, Polyporales) based on a five-marker dataset Alfredo Justo & David S. Hibbett Clark University, Biology Department, 950 Main St., Worcester, Massachusetts 01610, U.S.A. Author for correspondence: Alfredo Justo, [email protected] Abstract: The phylogeny of Trametes and related genera was studied using molecular data from ribosomal markers (nLSU, ITS) and protein-coding genes (RPB1, RPB2, TEF1-alpha) and consequences for the taxonomy and nomenclature of this group were considered. Separate datasets with rDNA data only, single datasets for each of the protein-coding genes, and a combined five-marker dataset were analyzed. Molecular analyses recover a strongly supported trametoid clade that includes most of Trametes species (including the type T. suaveolens, the T. versicolor group, and mainly tropical species such as T. maxima and T. cubensis) together with species of Lenzites and Pycnoporus and Coriolopsis polyzona. Our data confirm the positions of Trametes cervina (= Trametopsis cervina) in the phlebioid clade and of Trametes trogii (= Coriolopsis trogii) outside the trametoid clade, closely related to Coriolopsis gallica. The genus Coriolopsis, as currently defined, is polyphyletic, with the type species as part of the trametoid clade and at least two additional lineages occurring in the core polyporoid clade. In view of these results the use of a single generic name (Trametes) for the trametoid clade is considered to be the best taxonomic and nomenclatural option as the morphological concept of Trametes would remain almost unchanged, few new nomenclatural combinations would be necessary, and the classification of additional species (i.e., not yet described and/or sampled for mo- lecular data) in Trametes based on morphological characters alone will still be possible. -
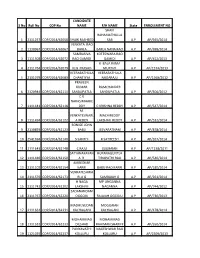
S No Roll No COP No CANDIDATE NAME F/H NAME State
CANDIDATE S No Roll No COP No NAME F/H NAME State ENROLLMENT NO SHAIK RAHAMATHULLA 1 2111257 COP/2014/62058 SHAIK RASHEED SAB A.P AP/945/2014 VENKATA RAO 2 1130967 COP/2014/62067 BARLA BARLA NANA RAO A.P AP/698/2014 SAMBASIVA KOTESWARA RAO 3 1111308 COP/2014/62072 RAO GAMIDI GAMIDI A.P AP/452/2013 K BALA RAMA 4 2111764 COP/2014/62079 KLN PRASAD MURTHY A.P AP/1574/2013 VEERABATHULA VEERABATHULA 5 2131079 COP/2014/62083 CHANTIYYA NAGARAJU A.P AP/1568/2012 PRAVEEN KUMAR RAMCHANDER 6 2120944 COP/2014/62111 SANDUPATLA SANDUPATLA A.P AP/306/2012 C V NARASIMHARE 7 1111441 COP/2014/62118 DDY C KRISHNA REDDY A.P AP/547/2014 M. VENKATESWARL MACHIREDDY 8 1111494 COP/2014/62122 A REDDY LAKSHMI REDDY A.P AP/532/2014 BONIGE JOHN 9 2130893 COP/2014/62123 BABU JEEVARATNAM A.P AP/878/2014 10 2541694 COP/2014/62140 S SANTHI R SATHEESH A.P AP/267/2014 11 2111643 COP/2014/62148 C RAJU SUGRAIAH A.P AP/1238/2011 SATYANARAYAN RUPANAGUNTLA 12 1111480 COP/2014/62150 A R. TIRUPATHI RAO A.P AP/540/2014 AMBEDKAR 13 2131102 COP/2014/62154 KARRI BABU RAO KARRI A.P AP/180/2014 VENKATESHWA 14 2111570 COP/2014/62173 RLU G SAMBAIAH G A.P AP/261/2014 H NAGA MP LINGANNA 15 2111742 COP/2014/62202 LAKSHMI NAGANNA A.P AP/744/2012 SADANANDAM 16 2111767 COP/2014/62220 OGGOJU RAJAIAH OGGOJU A.P AP/736/2013 MADHUSUDAN MOGILAIAH 17 2111661 COP/2014/62231 KACHAGANI KACHAGANI A.P AP/478/2014 MOHAMMAD MOHAMMAD 18 1111532 COP/2014/62233 DILSHAD RAHIMAN SHARIFF A.P AP/550/2014 PUNYAVATHI NAGESHWAR RAO 19 1121035 COP/2014/62237 KOLLURU KOLLURU A.P AP/2309/2013 G SATHAKOTI GEESALA 20 2131021 COP/2014/62257 SRINIVAS NAGABHUSHANAM A.P AP/1734/2011 GANTLA GANTLA SADHU 21 1131067 COP/2014/62258 SANYASI RAO RAO A.P AP/1802/2013 KOLICHALAM NAVEEN KOLICHALAM 22 1111688 COP/2014/62265 KUMAR BRAHMAIAH A.P AP/1908/2010 SRINIVASA RAO SANKARA RAO 23 2131012 COP/2014/62269 KOKKILIGADDA KOKKILIGADDA A.P AP/793/2013 24 2120971 COP/2014/62275 MADHU PILLI MAISAIAH PILLI A.P AP/108/2012 SWARUPARANI 25 2131014 COP/2014/62295 GANJI GANJIABRAHAM A.P AP/137/2014 26 2111507 COP/2014/62298 M RAVI KUMAR M LAXMAIAH A.P AP/177/2012 K. -

Caveats of Fungal Barcoding: a Case Study in Trametes S.Lat
Caveats of fungal barcoding: a case study in Trametes s.lat. (Basidiomycota: Polyporales) in Vietnam reveals multiple issues with mislabelled reference sequences and calls for third-party annotations Authors: Lücking, Robert, Truong, Ba Vuong, Huong, Dang Thi Thu, Le, Ngoc Han, Nguyen, Quoc Dat, et al. Source: Willdenowia, 50(3) : 383-403 Published By: Botanic Garden and Botanical Museum Berlin (BGBM) URL: https://doi.org/10.3372/wi.50.50302 BioOne Complete (complete.BioOne.org) is a full-text database of 200 subscribed and open-access titles in the biological, ecological, and environmental sciences published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Complete website, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/terms-of-use. Usage of BioOne Complete content is strictly limited to personal, educational, and non - commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research. Downloaded From: https://bioone.org/journals/Willdenowia on 10 Feb 2021 Terms of Use: https://bioone.org/terms-of-use Willdenowia Annals of the Botanic Garden and Botanical Museum Berlin ROBERT LÜCKING1*, BA VUONG TRUONG2, DANG THI THU HUONG3, NGOC HAN LE3, QUOC DAT NGUYEN4, VAN DAT NGUYEN5, ECKHARD VON RAAB-STRAUBE1, SARAH BOLLENDORFF1, KIM GOVERS1 & VANESSA DI VINCENZO1 Caveats of fungal barcoding: a case study in Trametes s.lat. -

Sporocarp Ontogeny in Panus (Basidiomycotina): Evolution and Classification
Sporocarp Ontogeny in Panus (Basidiomycotina): Evolution and Classification David S. Hibbett; Shigeyuki Murakami; Akihiko Tsuneda American Journal of Botany, Vol. 80, No. 11. (Nov., 1993), pp. 1336-1348. Stable URL: http://links.jstor.org/sici?sici=0002-9122%28199311%2980%3A11%3C1336%3ASOIP%28E%3E2.0.CO%3B2-M American Journal of Botany is currently published by Botanical Society of America. Your use of the JSTOR archive indicates your acceptance of JSTOR's Terms and Conditions of Use, available at http://www.jstor.org/about/terms.html. JSTOR's Terms and Conditions of Use provides, in part, that unless you have obtained prior permission, you may not download an entire issue of a journal or multiple copies of articles, and you may use content in the JSTOR archive only for your personal, non-commercial use. Please contact the publisher regarding any further use of this work. Publisher contact information may be obtained at http://www.jstor.org/journals/botsam.html. Each copy of any part of a JSTOR transmission must contain the same copyright notice that appears on the screen or printed page of such transmission. The JSTOR Archive is a trusted digital repository providing for long-term preservation and access to leading academic journals and scholarly literature from around the world. The Archive is supported by libraries, scholarly societies, publishers, and foundations. It is an initiative of JSTOR, a not-for-profit organization with a mission to help the scholarly community take advantage of advances in technology. For more information regarding JSTOR, please contact [email protected]. http://www.jstor.org Tue Jan 8 09:54:21 2008 American Journal of Botany 80(11): 1336-1348. -

Identification of Volatile Organic Compound Producing Lignicolous Fungal Cultures from Gujarat, India
Available online at www.worldscientificnews.com WSN 90 (2017) 150-165 EISSN 2392-2192 Identification of volatile organic compound producing Lignicolous fungal cultures from Gujarat, India Praveen Kumar Nagadesi1,*, Arun Arya2, Duvvi Naveen Babu3, K. S. M. Prasad3, P. P. Devi3 1Department of Botany, P.G. section, Andhra Loyola College, Vijayawada - 520008, Andhra Pradesh, India 2Department of Botany, Faculty of Science, The Maharaja Sayajirao University of Baroda, Vadodara - 390002, Gujarat, India 3St. Joseph Dental College, Dugirala, Eluru, Andhra Pradesh, India *E-mail address: [email protected] ABSTRACT This study aims to identify the lignicolous basidiomycetes species that synthetize volatile organic compounds with potential applications in food industry, cosmetics, perfumery and agriculture. We have collected fruiting bodies from different woody plants and the lignicolous basidiomyctes species were identified by their macroscopic and microscopic characteristics. From the context of the fresh fruiting bodies small fragments of dikaryotic mycelium were extracted and inoculated on PDA and MEA media for isolation and pure cultures are kept in dark at a temperature of 25°C. 11 species of lignicolous basidiomycetes, belonging to 6 families and 5 orders were isolated in pure culture. The isolates were analyzed in vitro and the main characteristics that were observed are: the general aspect of the surface and the reverse of the colonies, the changing in colour and the growth rate of the mycelium and also the specific odour which indicates the presence of the organic volatile compounds. for the first time lignicolous fungi like Flavodon flavus (Klotz.) Ryv., Ganoderma lucidum(Curtis) P. Karst, Hexagonia apiaria (Pers.) Fr., Lenzites betulina (L.) Fr. -

Wood-Rotting Fungi in East Khasi Hills of Meghalaya, Northeast India, with Special Reference to Heterobasidion Perplexa (A Rare Species ‒ New to India)
Current Research in Environmental & Applied Mycology 4 (1): 117–124 (2014) ISSN 2229-2225 www.creamjournal.org Article CREAM Copyright © 2014 Online Edition Doi 10.5943/cream/4/1/10 Wood-rotting fungi in East Khasi Hills of Meghalaya, northeast India, with special reference to Heterobasidion perplexa (a rare species ‒ new to India) Lyngdoh A1,2* and Dkhar MS1 1Microbial Ecology Laboratory, Department of Botany, North Eastern Hill University, Shillong- 793022, Meghalaya, India. 2Department of Botany, Shillong College, Shillong – 793003, Meghalaya, India. Email: [email protected] Lyngdoh A, Dkhar MS 2014 ‒ Wood-rotting fungi in East Khasi Hills of Meghalaya, Northeast India, with special reference to Heterobasidion perplexa (a rare species ‒ new to India). Current Research in Environmental & Applied Mycology 4 (1): 117–124, Doi 10.5943/cream/4/1/10 Abstract Field surveys and collection of the basidiocarps of wood-rotting fungi were carried out in eight forest stands of East Khasi Hills district of Meghalaya, India. Seventy eight wood-rotting fungi belonging to 23 families were identified. The undisturbed Mawphlang sacred grove was found to harbour a much larger number of the wood-rotting fungi (33.54 %) as compared to the other forest stands studied. Similarly, logs also harboured the maximum number of wood-rotting fungi (59.7 %) while living trees harboured the least (7.8%). Microporus xanthopus had the highest frequency percentage of occurrence with 87.5 %, followed by Cyclomyces tabacinus, Microporus affinis and Trametes versicolor with 62.5 %. Majority of the wood-rotting fungi are white-rot fungi (89.61%) and only few are brown-rots. -

Daedaleopsis Confragosa Daedaleopsis
© Demetrio Merino Alcántara [email protected] Condiciones de uso Daedaleopsis confragosa (Bolton) J. Schröt., in Cohn, Krypt.-Fl. Schlesien (Breslau) 3.1(25–32): 492 (1888) [1889] 20 mm 20 mm Polyporaceae, Polyporales, Incertae sedis, Agaricomycetes, Agaricomycotina, Basidiomycota, Fungi Sinónimos homotípicos: Boletus confragosus Bolton, Hist. fung. Halifax, App. (Huddersfield) 3: 160 (1792) [1791] Daedalea confragosa (Bolton) Pers., Syn. meth. fung. (Göttingen) 2: 501 (1801) Polyporus confragosus (Bolton) P. Kumm., Führ. Pilzk. (Zerbst): 59 (1871) Striglia confragosa (Bolton) Kuntze, Revis. gen. pl. (Leipzig) 2: 871 (1891) Lenzites confragosus (Bolton) Pat., Essai Tax. Hyménomyc. (Lons-le-Saunier): 89 (1900) Trametes confragosa (Bolton) Jørst., Atlas Champ. l'Europe, III, Polyporaceae (Praha) 1: 286 (1939) Ischnoderma confragosum (Bolton) Zmitr. [as 'confragosa'], Mycena 1(1): 92 (2001) Material estudiado: Francia, Aquitania, Osse en Aspe, Les Arrigaux, 30TXN8663, 931 m, en bosque de Abies sp. y Fagus sylvatica sobre madera sin determinar, 27-IX-2018, Dianora Estrada y Demetrio Merino, JA-CUSSTA: 9250. Descripción macroscópica: Carpóforo de 68 x 45 mm (alto x ancho), sésil, flabeliforme, con la cara externa rugosa, glabra, zonado concéntricamente con colo- res que van del blanquecino al marrón amarillento más o menos oscuro, margen agudo, blanco. Himenio en la cara inferior, delga- do, porado. Poros por lo general alargados, algunos redondeados, dispuestos en la dirección de los radios, que se manchan de marrón claro al roce, de (0,5-)0,8-2,0(-3,6) × (0,4-)0,5-0,8(-0,9) mm; N = 53; Me = 1,4 × 0,6 mm. Olor agradable, resinoso. Descripción microscópica: Basidios no observados. -

Short Title: Lentinus, Polyporellus, Neofavolus
In Press at Mycologia, preliminary version published on February 6, 2015 as doi:10.3852/14-084 Short title: Lentinus, Polyporellus, Neofavolus Phylogenetic relationships and morphological evolution in Lentinus, Polyporellus and Neofavolus, emphasizing southeastern Asian taxa Jaya Seelan Sathiya Seelan Biology Department, Clark University, 950 Main Street, Worcester, Massachusetts 01610, and Institute for Tropical Biology and Conservation (ITBC), Universiti Malaysia Sabah, 88400 Kota Kinabalu, Sabah, Malaysia Alfredo Justo Laszlo G. Nagy Biology Department, Clark University, 950 Main Street, Worcester, Massachusetts 01610 Edward A. Grand Mahidol University International College (Science Division), 999 Phuttamonthon, Sai 4, Salaya, Nakorn Pathom 73170, Thailand Scott A. Redhead ECORC, Science & Technology Branch, Agriculture & Agri-Food Canada, CEF, Neatby Building, Ottawa, Ontario, K1A 0C6 Canada David Hibbett1 Biology Department, Clark University, 950 Main Street Worcester, Massachusetts 01610 Abstract: The genus Lentinus (Polyporaceae, Basidiomycota) is widely documented from tropical and temperate forests and is taxonomically controversial. Here we studied the relationships between Lentinus subg. Lentinus sensu Pegler (i.e. sections Lentinus, Tigrini, Dicholamellatae, Rigidi, Lentodiellum and Pleuroti and polypores that share similar morphological characters). We generated sequences of internal transcribed spacers (ITS) and Copyright 2015 by The Mycological Society of America. partial 28S regions of nuc rDNA and genes encoding the largest subunit of RNA polymerase II (RPB1), focusing on Lentinus subg. Lentinus sensu Pegler and the Neofavolus group, combined these data with sequences from GenBank (including RPB2 gene sequences) and performed phylogenetic analyses with maximum likelihood and Bayesian methods. We also evaluated the transition in hymenophore morphology between Lentinus, Neofavolus and related polypores with ancestral state reconstruction. -

Anti-Staphylococcal and Antioxidant Properties of Crude Ethanolic Extracts of Macrofungi Collected from the Philippines
Pharmacogn J. 2018; 10(1):106-109 A Multifaceted Journal in the field of Natural Products and Pharmacognosy Original Article www.phcogj.com | www.journalonweb.com/pj | www.phcog.net Anti-Staphylococcal and Antioxidant Properties of Crude Ethanolic Extracts of Macrofungi Collected from the Philippines Christine May Gaylan1, John Carlo Estebal1, Ourlad Alzeus G. Tantengco2, Elena M. Ragragio1 ABSTRACT Introduction: Macrofungi have been used in the Philippines as source of food and traditional medicines. However, these macrofungi in the Philippines have not yet been studied for different biological activities. Thus, this research determined the potential antibacterial and antioxidant activities of crude ethanolic extracts of seven macrofungi collected in Bataan, Philippines. Methods: Kirby-Bauer disk diffusion assay and broth microdilution method were used to screen for the antibacterial activity and DPPH scavenging assay for the determination of antioxidant activity. Results: F. rosea, G. applanatum, G. lucidum and P. pinisitus exhibited zones of inhibition ranging from 6.55 ± 0.23 mm to 7.43 ± 0.29 mm against S. aureus, D. confragosa, F. rosea, G. lucidum, M. xanthopus and P. pinisitus showed antimicrobial activities against S. aureus with an MIC50 ranging from 1250 μg/mL to 10000 μg/mL. F. rosea, G. applanatum, G. lucidum, M. xanthopus exhibited excellent antioxidant activity with F. rosea having the highest antioxidant activity among all the extracts tested (3.0 μg/mL). Conclusion: Based on the results, these Philippine macrofungi showed antistaphylococcal activity independent of 1 Christine May Gaylan , the antioxidant activity. These can be further studied as potential sources of antibacterial and John Carlo Estebal1, antioxidant compounds.