In the Cape to Cape Region Protecting Aquatic Biodiversity
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Assessment of Wetland Invertebrate and Fish Biodiversity for the Gnangara Sustainability Strategy (Gss)
ASSESSMENT OF WETLAND INVERTEBRATE AND FISH BIODIVERSITY FOR THE GNANGARA SUSTAINABILITY STRATEGY (GSS) Bea Sommer, Pierre Horwitz and Pauline Hewitt Centre for Ecosystem Management Edith Cowan University, Joondalup WA 6027 Final Report to the Western Australian Department of Environment and Conservation November 2008 Assessment of wetland invertebrate and fish biodiversity for the GSS (Final Report) November 2008 This document has been commissioned/produced as part of the Gnangara Sustainability Strategy (GSS). The GSS is a State Government initiative which aims to provide a framework for a whole of government approach to address land use and water planning issues associated with the Gnangara groundwater system. For more information go to www.gnangara.water.wa.gov.au i Assessment of wetland invertebrate and fish biodiversity for the GSS (Final Report) November 2008 Executive Summary This report sought to review existing sources of information for aquatic fauna on the Gnangara Mound in order to: • provide a synthesis of the richness, endemism, rarity and habitat specificity of aquatic invertebrates in wetlands; • identify gaps in aquatic invertebrate data on the Gnangara Mound; • provide a synthesis of the status of freshwater fishes on the Gnangara Mound; • assess the management options for the conservation of wetlands and wetland invertebrates. The compilation of aquatic invertebrate taxa recorded from wetlands on both the Gnangara Mound and Jandakot Mound) between 1977 and 2003, from 18 studies of 66 wetlands, has revealed a surprisingly high richness considering the comparatively small survey area and the degree of anthropogenic alteration of the plain. The total of over 550 taxa from 176 families or higher order taxonomic levels could be at least partially attributed to sampling effort. -

Coastal Land and Groundwater for Horticulture from Gingin to Augusta
Research Library Resource management technical reports Natural resources research 1-1-1999 Coastal land and groundwater for horticulture from Gingin to Augusta Dennis Van Gool Werner Runge Follow this and additional works at: https://researchlibrary.agric.wa.gov.au/rmtr Part of the Agriculture Commons, Natural Resources Management and Policy Commons, Soil Science Commons, and the Water Resource Management Commons Recommended Citation Van Gool, D, and Runge, W. (1999), Coastal land and groundwater for horticulture from Gingin to Augusta. Department of Agriculture and Food, Western Australia, Perth. Report 188. This report is brought to you for free and open access by the Natural resources research at Research Library. It has been accepted for inclusion in Resource management technical reports by an authorized administrator of Research Library. For more information, please contact [email protected], [email protected], [email protected]. ISSN 0729-3135 May 1999 Coastal Land and Groundwater for Horticulture from Gingin to Augusta Dennis van Gool and Werner Runge Resource Management Technical Report No. 188 LAND AND GROUNDWATER FOR HORTICULTURE Information for Readers and Contributors Scientists who wish to publish the results of their investigations have access to a large number of journals. However, for a variety of reasons the editors of most of these journals are unwilling to accept articles that are lengthy or contain information that is preliminary in nature. Nevertheless, much material of this type is of interest and value to other scientists, administrators or planners and should be published. The Resource Management Technical Report series is an avenue for the dissemination of preliminary or lengthy material relevant the management of natural resources. -

82452 JW.Rdo
Item 9.1.19 Item 9.1.19 Item 9.1.19 Item 9.1.19 Item 9.1.19 Item 9.1.19 Item 9.1.19 Item 9.1.19 WSD Item 9.1.19 H PP TONKIN HS HS HWY SU PICKERING BROOK HS ROE HS TS CANNING HILLS HS HWY MARTIN HS HS SU HS GOSNELLS 5 8 KARRAGULLEN HWY RANFORD HS P SOUTHERN 9 RIVER HS 11 BROOKTON SU 3 ROAD TS 12 H ROLEYSTONE 10 ARMADALE HWY 13 HS ROAD 4 WSD ARMADALE 7 6 FORRESTDALE HS 1 ALBANY 2 ILLAWARRA WESTERN BEDFORDALE HIGHWAY WSD THOMAS ROAD OAKFORD SOUTH WSD KARRAKUP OLDBURY SU Location of the proposed amendment to the MRS for 1161/41 - Parks and Recreation Amendment City of Armadale METROPOLITAN REGION SCHEME LEGEND Proposed: RESERVED LANDS ZONES PARKS AND RECREATION PUBLIC PURPOSES - URBAN Parks and Recreation Amendment 1161/41 DENOTED AS FOLLOWS : 1 R RESTRICTED PUBLIC ACCESS URBAN DEFERRED City of Armadale H HOSPITAL RAILWAYS HS HIGH SCHOOL CENTRAL CITY AREA TS TECHNICAL SCHOOL PORT INSTALLATIONS INDUSTRIAL CP CAR PARK U UNIVERSITY STATE FORESTS SPECIAL INDUSTRIAL CG COMMONWEALTH GOVERNMENT WATER CATCHMENTS SEC STATE ENERGY COMMISSION RURAL SU SPECIAL USES CIVIC AND CULTURAL WSD WATER AUTHORITY OF WA PRIVATE RECREATION P PRISON WATERWAYS RURAL - WATER PROTECTION ROADS : PRIMARY REGIONAL ROADS METROPOLITAN REGION SCHEME BOUNDARY OTHER REGIONAL ROADS armadaleloc.fig N 26 Mar 2009 Produced by Mapping & GeoSpatial Data Branch, Department for Planning and Infrastructure Scale 1:150 000 On behalf of the Western Australian Planning Commission, Perth WA 0 4 Base information supplied by Western Australian Land Information Authority GL248-2007-2 GEOCENTRIC -

Atic Fa and T Ralia's Sout
Aquatic fauna refuges in Marrggaret River and the Cape to Cape region of Australia’s Mediterranean-climatic Southwestern Province Mark G. Allen1,2, Stephen J. Beatty1 and David L. Morgan1* 1. Freshwater Fish Group & Fish Health Unit, Centre for Fish & Fisheries Research, School of Veterinary and Life Sciences, Murdoch University, South St, Murdoch 6150, Western Austrralia 2. Current address: Department of Aquattic Zoology, Western Australian Museum, Locked Bag 49, Welshpool DC, Perth, Western Australia, 6986, Australia * correspondence to [email protected] SUUMMARY Margaret River and the Cape to Cape region in the extreme south-western tip of Australia are located between Capee Naturaliste in the north and Cape Leeuwin in the south and encompass all intervening catchments that drain westward to the In- dian Ocean. The region has a Mediteerranean climate and houses 13 native, obligate freshwater macrofauna species (i.e. fishes, decapod crustaceans and a bivalve mol- lusc), four of which are listed as threatened under State and/or Commonwealth leg- islation. The most imperiled species are the Margaret River Burrowing Crayfish (Engaewa pseudoreducta) and Hairy Marron (Cherax tenuimanus), both of which are endemic to the Margaret River catchment and listed as critically endangered (also by the IUCN), and Balston’s Pygmy Perch (Nannatherina balstoni) which is vulner- able. The region also houses several fishes that may represent neew, endemic taxa based on preliminary molecular evidence. Freshwater ecosystems in the region face numerous threats including global climate change, a growing huuman population, introduced species, destructive land uses, riparian degradation, waater abstraction, declinning environmental flows, instream barriers, and fire. -

Final Annual Report 2005-2006
About us Contents MINISTER FOR THE Executive Director’s review 2 ENVIRONMENT About us 4 In accordance with Our commitment 4 Section 70A of the Our organisation 7 Financial Administration The year in summary 12 and Audit Act 1985, I submit for your Highlights of 2005-2006 12 Strategic Planning Framework 16 information and presentation to Parliament What we do 18 the final annual report of Nature Conservation – Service 1 18 the Department of Sustainable Forest Management – Service 2 65 Conservation and Land Performance of Statutory Functions by the Conservation Commission Management. of Western Australia (see page 194) – Service 3 Parks and Visitor Services – Service 4 76 Astronomical Services – Service 5 112 General information 115 John Byrne Corporate Services 115 REPORTING CALM-managed lands and waters 118 OFFICER Estate map 120 31 August 2006 Fire management services 125 Statutory information 137 Public Sector Standards and Codes of Conduct 137 Legislation 138 Disability Services 143 EEO and diversity management 144 Electoral Act 1907 145 Energy Smart 146 External funding, grants and sponsorships 147 Occupational safety and health 150 Record keeping 150 Substantive equality 151 Waste paper recycling 151 Publications produced in 2005-2006 152 Performance indicators 174 Financial statements 199 The opinion of the Auditor General appears after the performance indicators departmentofconservationandlandmanagement 1 About us Executive Director’s review The year in review has proved to be significant for the Department of Conservation and Land Management (CALM) for the work undertaken and because it has turned out to be the Department’s final year of operation. The Minister for the Environment announced in May 2006 that CALM would merge with the Department of Environment on 1 July 2006 to form the Department of Environment and Conservation. -

Dunsborough Burrowing Crayfish (Engaewa Reducta), Margaret River
Dunsborough Burrowing Crayfish (Engaewa reducta), Margaret River Burrowing Crayfish (Engaewa pseudoreducta) and Walpole Burrowing Crayfish (Engaewa walpolea) Recovery Plan 2007 –2016 Wildlife Management Program No. 41 WESTERN AUSTRALIAN WILDLIFE MANAGEMENT PROGRAM NO. 41 Dunsborough Burrowing Crayfish (Engaewa reducta), Margaret River Burrowing Crayfish (Engaewa pseudoreducta) and Walpole Burrowing Crayfish (Engaewa walpolea) Recovery Plan 2007-2016 23 January 2008 Species and Communities Branch Department of Conservation and Land Management Locked Bag 104, Bentley Delivery Centre WA 6983 Above: Dunsborough Burrowing Crayfish (Engaewa reducta) Photo:Kelly Rogerson Cover: Walpole Burrowing Crayfish (Engaewa walpolea) Photo:Kelly Rogerson i. FOREWORD Recovery Plans are developed within the framework laid down in Department of Conservation and Land Management (CALM) Policy Statements No 44 and 50. Recovery Plans outline the recovery actions that are required to address those threatening processes most affecting the ongoing survival of threatened taxa or ecological communities, and begin the recovery process. Recovery Plans delineate, justify and schedule management actions necessary to support the recovery of threatened species and ecological communities. The attainment of objectives and the provision of funds necessary to implement actions are subject to budgetary and other constraints affecting the parties involved, as well as the need to address other priorities. Recovery Plans do not necessarily represent the views or the official position of individuals or organisations represented on the Recovery Team. This Recovery Plan was approved by the Department of Environment and Conservation, Western Australia. Approved Recovery Plans are subject to modification as dictated by new findings, changes in status of the taxon or ecological community and the completion of recovery actions. -

Aquatic Invertebrate Fauna of the Two Peoples Bay Area, Southwestern Australia
Journal of the Royal Society of Westem Australia, 76: 25-32, 1993 Aquatic invertebrate fauna of the Two Peoples Bay area, southwestern Australia 14, 15 2R W Storey, A Halse & J Shiel lDePartment of Conservation and Land Managemen! Wildlife Research Centre, PO Box 51, Wanneroo WA 6065 2Murray-Darling Freshwater Research Centre, PO Box 921, Albury NSW 2640 Ma usctipt receioed luly 1992; accepted Decenbet 1992 Abstract The aquatic invertebrate fauna of four rivers and streams and three lakes in the Two Peoples Bay area, southwestern Australia, was sampled in June 1990 and February 1991. A total of 247 taxa were recbrded: ll0 in flowing waters, 170 in standing walers and 33 common to bolh habitats. lncreased sampling intensity in February 1991 revealed a diverse microinvertebrate fauna of 47 ta\a of Protozoa and Rotifera. The macroinvertebrate fauna was similar to that reDorted previouslv from aouatic eco\vstems in southwestern Austratia. fh irty-si^ species of m icroinvertebrates 1) Protozoi, 2 I Rotifdra, 10 Chdocera and 3 Copepodal were new records for southwestern Australia and, in many cdses, Austra[a. The number of new records reflects greater sampling and taxonomic effort than has been used previously in southwestern Australia, but also suggests southwestern Australia may have a distinctive miirofauna.' Introduction The Coodga system comprises the Goodgd River, Moates Lake and Cardner Lake and enters the setin Two Peoples Peoples Two Bay is an important area for wildlife Bav. The slow-flowing Goodga fuver arises approximaiely conservation. It is best known as the last refuge for the 20-km inland. ln its u[per reiches, the river fid.s thro,lgir Noisy Scrub-bird Atricholnis clamosus and a nature reserve cleared farmland before entering the reserve and dischary- was proclaimed in 7966 to protect this species. -

Appendix 5 Baseline Aquatic Biology and Water Quality Study
Cloverdale Project M-CV-00028 Baseline Aquatic Biology and Water Quality Study including Tiger Gully, Ludlow River and Capel River prepared for by Wetland Research & Management Cloverdale Project M-CV-00028 Baseline Aquatic Biology and Water Quality Study including Tiger Gully, Ludlow River and Capel River Prepared for: Iluka Resources Limited Level 23, 140 St Georges Terrace, Perth WA 6000 GPO Box U1988 Perth WA Ph (61 8) 9360 4700 By: Wetland Research & Management 28 William Street, Glen Forrest, WA 6071, Australia Ph (61 8) 9298 9807, Fax (61 8) 9380 1029, e-mail: awstorey@ cyllene.uwa.edu.au Draft Report April 2006 Frontispiece: (clockwise from main picture) Ludlow River, 1.2 km downstream from the Cloverdale project area; male koonac Cherax plebejus; nightfish Bostockia porosa. ii Study Team 8anagement: Sue Creagh Field Work: Sue Creagh & Jess Lynas Macroinvertebrate Identification: Lisa Chandler & Sue Creagh Data analysis: Sue Creagh & Andrew Storey Report: Sue Creagh Acknowledgements This project was undertaken by Wetland Research & Management (WRM) for Iluka Resources Limited. WRM would like to acknowledge Dr Don Edward (UWA) for assistance with Chironomidae taxonomy and Dr Mark Harvey (WAM) for Acarina taxonomy and Dr Rob Davis (Western Wildlife) for tadpole identifications. The maps of the study area were provided by Iluka Resources Limited. Shannon Jones (Iluka) is thanked for constructive criticism on the draft report and for her efficient overall management of this project on behalf of Iluka. The authors are grateful to Craig and Tom Hutton, Garry Bibby, Jan McKechnie and to the Norton, Armstrong, Weir and Whiteland families, who readily granted access to their pastoral properties. -

Wellington National Park, Westralia Conservation Park and Wellington Discovery Forest
WELLINGTON NATIONAL PARK, WESTRALIA CONSERVATION PARK AND WELLINGTON DISCOVERY FOREST Management Plan 2008 Department of Environment and Conservation Conservation Commission of Western Australia VISION Over the life of the plan, a balance will exist between the conservation of the planning areas’ natural values and the public demand for recreation and water supply. The area will make an important contribution to reservation of the Jarrah Forest, where natural values, such as granite outcrops, mature growth forest, ecosystems of the Collie River, and our knowledge of them, will be maintained and enhanced for future generations. Visitors to the area will enjoy a range of sustainable recreation opportunities in a variety of forest settings, and provide a benefit to the regional economy. The community will regard the area as a natural asset and will have a greater understanding of its values, and support for their management, through the Wellington Discovery Forest and other education and interpretive facilities. The ancient landscape of the Collie River valley will be recognised as a forest environment of great visual aesthetic appeal, and for its rich Aboriginal heritage, which will be kept alive through the active and ongoing involvement of local Aboriginal people. ii PREFACE The Department of Environment and Conservation (the Department) manages reserves vested in the Conservation Commission of Western Australia (Conservation Commission) and prepares management plans on their behalf. The Conservation Commission issues draft management plans for public comment and provides proposed (final) management plans for approval by the Minister for the Environment. The Conservation and Land Management Act 1984 (the ‘CALM Act’) specifies that management plans must contain: a) a statement of policies and guidelines proposed to be followed; and b) a summary of operations proposed to be undertaken. -

Flood Hydrograph Estimation
A GUI D E TO F LOOD ESTIMA TIO N BOOK 5 - FLOOD HYDROGRAPH ESTIMATION The Australian Rainfall and Runoff: A guide to flood estimation (ARR) is licensed under the Creative Commons Attribution 4.0 International Licence, unless otherwise indicated or marked. Please give attribution to: © Commonwealth of Australia (Geoscience Australia) 2019. Third-Party Material The Commonwealth of Australia and the ARR’s contributing authors (through Engineers Australia) have taken steps to both identify third-party material and secure permission for its reproduction and reuse. However, please note that where these materials are not licensed under a Creative Commons licence or similar terms of use, you should obtain permission from the relevant third-party to reuse their material beyond the ways you are legally permitted to use them under the fair dealing provisions of the Copyright Act 1968. If you have any questions about the copyright of the ARR, please contact: [email protected] c/o 11 National Circuit, Barton, ACT ISBN 978-1-925848-36-6 How to reference this book: Ball J, Babister M, Nathan R, Weeks W, Weinmann E, Retallick M, Testoni I, (Editors) Australian Rainfall and Runoff: A Guide to Flood Estimation, © Commonwealth of Australia (Geoscience Australia), 2019. How to reference Book 9: Runoff in Urban Areas: Coombes, P., and Roso, S. (Editors), 2019 Runoff in Urban Areas, Book 9 in Australian Rainfall and Runoff - A Guide to Flood Estimation, Commonwealth of Australia, © Commonwealth of Australia (Geoscience Australia), 2019. PREFACE Since its first publication in 1958, Australian Rainfall and Runoff (ARR) has remained one of the most influential and widely used guidelines published by Engineers Australia (EA). -

Northern Territory
NORTHERN TERRITORY BAYVIEW 0820 CHARLES DARWIN 0820 COONAWARRA 0820 CULLEN BAY 0820 DARWIN INTERNATIONAL AIRPORT 0820 EAST POINT 0820 EATON 0820 FANNIE BAY 0820 LARRAKEYAH 0820 LUDMILLA 0820 PARAP 0820 RAAF BASE DARWIN 0820 STUART PARK 0820 THE GARDENS 0820 THE NARROWS 0820 WINNELLIE 0820 WOOLNER 0820 BAGOT 0820 DARWIN DC 0820 DARWIN MC 0820 WINNELLIE 0821 ACACIA HILLS 0822 ANGURUGU 0822 ANINDILYAKWA 0822 ANNIE RIVER 0822 BEES CREEK 0822 BELYUEN 0822 BLACK JUNGLE 0822 BLACKMORE 0822 BORDER STORE 0822 BURRUNDIE 0822 BYNOE 0822 BYNOE HARBOUR 0822 CAMP CREEK 0822 CHANNEL ISLAND 0822 CHARLES DARWIN 0822 CHARLOTTE 0822 CLARAVALE 0822 COBOURG 0822 COLLETT CREEK 0822 COOMALIE CREEK 0822 COX PENINSULA 0822 DALY 0822 DALY RIVER 0822 DARWIN RIVER DAM 0822 DELISSAVILLE 0822 DOUGLAS-DALY 0822 EAST ARM 0822 EAST ARNHEM 0822 ELRUNDIE 0822 EVA VALLEY 0822 FINNISS VALLEY 0822 FLEMING 0822 FLY CREEK 0822 FREDS PASS 0822 GALIWINKU 0822 GLYDE POINT 0822 GUNBALANYA 0822 GUNN POINT 0822 HAYES CREEK 0822 HIDDEN VALLEY 0822 HOTHAM 0822 HUGHES 0822 KAKADU 0822 KOOLPINYAH 0822 LAKE BENNETT 0822 LAMBELLS LAGOON 0822 LITCHFIELD PARK 0822 LIVINGSTONE 0822 LLOYD CREEK 0822 MANDORAH 0822 MANINGRIDA 0822 MAPURU 0822 MARANUNGA 0822 MARGARET RIVER 0822 MARRAKAI 0822 MCMINNS LAGOON 0822 MICKETT CREEK 0822 MIDDLE POINT 0822 MILIKAPITI 0822 MILINGIMBI 0822 MILYAKBURRA 0822 MINJILANG 0822 MOUNT BUNDEY 0822 MURRUMUJUK 0822 NAUIYU 0822 NEMARLUK 0822 NGANMARRIYANGA 0822 NUMBULWAR 0822 NUMBURINDI 0822 OENPELLI 0822 PALUMPA 0822 PEPPIMENARTI 0822 PIRLANGIMPI 0822 POINT STUART -
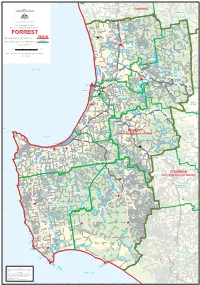
Map of the Division of Forrest
FORREST Lake Clifton Waroona OLD FORREST Yalgorup National WAROONA Hamel State Forest 2008 Park COAST CANNING COMMONWEALTH OF AUSTRALIA Hakea 20 BODDINGTON Yalgorup South Yalup Wagerup Willowdale Lower Dam Preston Beach SOUTH Yalup Brook Murray Williams Mt William RD Commonwealth Electoral Act 1918 River Yarloop River RD WESTERN River ELLIS STATE OF WESTERN AUSTRALIA Brook Map of the Commonwealth Electoral Division of Lake Brockman Bell Cookernup Harvey Moojelup Hoffman Lane Poole Reserve FORREST Harvey River Flats HWY Yamballup Harvey Names and Boundaries of Electoral Divisions PERTH 1 Warawarrup River Lake Preston KWINANA Korljekup Harvey Dam Names and Boundaries of Local Government Areas Harvey Tallanalla Map Number WA06/2008 20 Nalyern Lake Querilyup Stirling Dam Wokalup HARVEY Treesville Kilometres 0 5 10 15 Kilometres Harris Myalup River Wellesley River State Forest The Stinkwoods Flats Mornington SIMPLE CONIC PROJECTION WITH STANDARD PARALLELS 18° AND 36°S Yourdamung Lake Sheet 1 of 1 Binningup COLLIE Benger Lake Ballingall River RD Marbalup River Bingham Burragenup Beela Wellesley Brunswick Collie COLLIE Leschenault INDIAN OCEAN Hamilton - Fernbrook Creek Brunswick Junction WILLIAMS HWY Leschenault River Estuary Worsley Collie HWY Roelands River COALFIELDS River BYPASS Australind BYPASS 107 Cheetarra Allanson Gilfillan East Shenton Moorhead Ewington Collie Koombana Burekup Elbow Bay WESTERN COALFIELDS Eaton Waterloo Collie Buckingham Wilson Park Shotts BUNBURY River AUSTRALINDSOUTH RD 20 Collie Burn Cowcher 107 Wellington Dam