Rotating Arrays of Orchid Flowers: a Simple and Effective Method for Studying Pollination in Food Deceptive Plants
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

List of Plants Observed and Identified by Members of the Ringwood Field Naturalists Club Inc
LIST OF PLANTS OBSERVED AND IDENTIFIED BY MEMBERS OF THE RINGWOOD FIELD NATURALISTS CLUB INC. ON THEIR SPRING FIELD TRIP TO YARRAM 16-18 NOVEMBER 2012 COMPILED BY JUDITH V COOKE Ninety Mile Beach 17 11 2012 1 Map of Yarram and surrounding places visited 16-18 11 2012 2 3 Botanical Name Common Name 16 17 18 11 11 11 ORCHIDS Caladenia carnea Pink Fingers F Caladenia gracilis Musky Caladenia F Caleana major Flying Duck Orchid F Calochilus campestris Copper Beard Orchid F Chiloglottis cornuta Little Bird Orchid F Chiloglottis valida Common Bird Orchid F Diuris sulphurea Hornet Orchid F F Microtis sp Onion Orchid F Stegostyla transitoria Eastern Bronze Caladenia F Thelymitra ixiodes Spotted Sun Orchid F Thelymitra media Tall Sun Orchid F 4 5 Botanical Name Common Name 16 17 18 11 11 11 FERNS Asplenium bulbiferum Mother Spleenwort Blechnum chambersii Lance Water Fern Blechnum fluviatile Ray Water Fern Blechnum nudum Fishbone Water Fern Blechnum patersonii Strap Water Fern Blechnum wattsii Hard Water Fern Calochlaena dubia Common Ground Fern Cyathea australis Rough Tree Fern Dicksonia antarctica Soft Tree Fern Grammitis billardieri Finger Fern Histiopteris incisa Batswing Fern Hymenophyllum cupressiforme Common Filmy Fern Hymenophyllum sp? Filmy Fern Hymenophyllum sp? Filmy/Bristle Fern Lastreopsis acuminata Shiny Shield Fern Microsorum diversifolium Kangaroo Fern Polystichum proliferum Mother Shield Fern Pteridium esculentum Austral Bracken Rumohra adiantiformis Shield Hare's-foot 6 Botanical Name Common Name 16 17 18 -

Actes Du 15E Colloque Sur Les Orchidées De La Société Française D’Orchidophilie
Cah. Soc. Fr. Orch., n° 7 (2010) – Actes 15e colloque de la Société Française d’Orchidophilie, Montpellier Actes du 15e colloque sur les Orchidées de la Société Française d’Orchidophilie du 30 mai au 1er juin 2009 Montpellier, Le Corum Comité d’organisation : Daniel Prat, Francis Dabonneville, Philippe Feldmann, Michel Nicole, Aline Raynal-Roques, Marc-Andre Selosse, Bertrand Schatz Coordinateurs des Actes Daniel Prat & Bertrand Schatz Affiche du Colloque : Conception : Francis Dabonneville Photographies de Francis Dabonneville & Bertrand Schatz Cahiers de la Société Française d’Orchidophilie, N° 7, Actes du 15e Colloque sur les orchidées de la Société Française d’Orchidophilie. ISSN 0750-0386 © SFO, Paris, 2010 Certificat d’inscription à la commission paritaire N° 55828 ISBN 978-2-905734-17-4 Actes du 15e colloque sur les Orchidées de la Société Française d’Orchidophilie, D. Prat et B. Schatz, Coordinateurs, SFO, Paris, 2010, 236 p. Société Française d’Orchidophilie 17 Quai de la Seine, 75019 Paris Cah. Soc. Fr. Orch., n° 7 (2010) – Actes 15e colloque de la Société Française d’Orchidophilie, Montpellier Préface Ce 15e colloque marque le 40e anniversaire de notre société, celle-ci ayant vu le jour en 1969. Notre dernier colloque se tenait il y a 10 ans à Paris en 1999, 10 ans c’est long, 10 ans c’est très loin. Il fallait que la SFO renoue avec cette traditionnelle organisation de colloques, manifestation qui a contribué à lui accorder la place prépondérante qu’elle occupe au sein des orchidophiles français et de la communauté scientifique. C’est chose faite aujourd’hui. Nombreux sont les thèmes qui font l’objet de communications par des intervenants dont les compétences dans le domaine de l’orchidologie ne sont plus à prouver. -

Newsletter No
Newsletter No. 167 June 2016 Price: $5.00 AUSTRALASIAN SYSTEMATIC BOTANY SOCIETY INCORPORATED Council President Vice President Darren Crayn Daniel Murphy Australian Tropical Herbarium (ATH) Royal Botanic Gardens Victoria James Cook University, Cairns Campus Birdwood Avenue PO Box 6811, Cairns Qld 4870 Melbourne, Vic. 3004 Australia Australia Tel: (+61)/(0)7 4232 1859 Tel: (+61)/(0) 3 9252 2377 Email: [email protected] Email: [email protected] Secretary Treasurer Leon Perrie John Clarkson Museum of New Zealand Te Papa Tongarewa Queensland Parks and Wildlife Service PO Box 467, Wellington 6011 PO Box 975, Atherton Qld 4883 New Zealand Australia Tel: (+64)/(0) 4 381 7261 Tel: (+61)/(0) 7 4091 8170 Email: [email protected] Mobile: (+61)/(0) 437 732 487 Councillor Email: [email protected] Jennifer Tate Councillor Institute of Fundamental Sciences Mike Bayly Massey University School of Botany Private Bag 11222, Palmerston North 4442 University of Melbourne, Vic. 3010 New Zealand Australia Tel: (+64)/(0) 6 356- 099 ext. 84718 Tel: (+61)/(0) 3 8344 5055 Email: [email protected] Email: [email protected] Other constitutional bodies Hansjörg Eichler Research Committee Affiliate Society David Glenny Papua New Guinea Botanical Society Sarah Matthews Heidi Meudt Advisory Standing Committees Joanne Birch Financial Katharina Schulte Patrick Brownsey Murray Henwood David Cantrill Chair: Dan Murphy, Vice President Bob Hill Grant application closing dates Ad hoc adviser to Committee: Bruce Evans Hansjörg Eichler Research -

Intro Outline
THE REPRODUCTIVE ECOLOGY OF TWO TERRESTRIAL ORCHIDS, CALADENIA RIGIDA AND CALADENIA TENTACULATA RENATE FAAST Submitted for the degree of Doctor of Philosophy School of Earth and Environmental Sciences The University of Adelaide, South Australia December, 2009 i . DEcLARATION This work contains no material which has been accepted for the award of any other degree or diploma in any university or other tertiary institution to Renate Faast and, to the best of my knowledge and belief, contains no material previously published or written by another person, except where due reference has been made in the text. I give consent to this copy of my thesis when deposited in the University Library, being made available for loan and photocopying, subject to the provisions of the Copyright Act 1968. The author acknowledges that copyright of published works contained within this thesis (as listed below) resides with the copyright holder(s) of those works. I also give permission for the digital version of my thesis to be made available on the web, via the University's digital research repository, the Library catalogue, the Australasian Digital Theses Program (ADTP) and also through web search engines. Published works contained within this thesis: Faast R, Farrington L, Facelli JM, Austin AD (2009) Bees and white spiders: unravelling the pollination' syndrome of C aladenia ri gída (Orchidaceae). Australian Joumal of Botany 57:315-325. Faast R, Facelli JM (2009) Grazrngorchids: impact of florivory on two species of Calademz (Orchidaceae). Australian Journal of Botany 57:361-372. Farrington L, Macgillivray P, Faast R, Austin AD (2009) Evaluating molecular tools for Calad,enia (Orchidaceae) species identification. -

Daviesia Physodes LC Taxonomic Authority: A.Cunn
Daviesia physodes LC Taxonomic Authority: A.Cunn. ex G.Don Global Assessment Regional Assessment Region: Global Endemic to region Synonyms Common Names PRICKLY BITTER PEA English Upper Level Taxonomy Kingdom: PLANTAE Phylum: TRACHEOPHYTA Class: MAGNOLIOPSIDA Order: FABALES Family: LEGUMINOSAE Lower Level Taxonomy Rank: Infra- rank name: Plant Hybrid Subpopulation: Authority: General Information Distribution Daviesia physodes is endemic to Australia, distributed in the state of Western Australia. Range Size Elevation Biogeographic Realm Area of Occupancy: Upper limit: 380 Afrotropical Extent of Occurrence: Lower limit: Antarctic Map Status: Depth Australasian Upper limit: Neotropical Lower limit: Oceanian Depth Zones Palearctic Shallow photic Bathyl Hadal Indomalayan Photic Abyssal Nearctic Population Total population size and dynamics are not known, but a recent survey in 2005 suggests 100 plants from a population in Western Australia (MSBP 2010) growing in dense low heath. Total Population Size Minimum Population Size: Maximum Population Size: Habitat and Ecology This large shrub grows on sandy soils over laterite or limestone in plains, hills and winter-wet flats of Western Australia. It grows in woodlands, shrublands or heath. System Movement pattern Crop Wild Relative Terrestrial Freshwater Nomadic Congregatory/Dispersive Is the species a wild relative of a crop? Marine Migratory Altitudinally migrant Growth From Definition Shrub - large Perennial shrub (>1m), also termed a Phanerophyte (>1m) Threats This species is susceptible to Phytophthora cinnamomi, the jarrah dieback pathogen (Groves et al. 2009). This is a serious threat to the forest areas which caused the death of thousands of hectares of forest in this area (Boden and Given 1995). It is recommended that D. -

Vegetaton and Flora of Lot 9503 Wedgetail Circle Parkerville
VEGETATON AND FLORA OF LOT 9503 WEDGETAIL CIRCLE PARKERVILLE Prepared for: COTERRA ENVIRONMENT 19/336 Churchill Avenue, SUBIACO WA 6008 Prepared by: Bennett Environmental Consulting Pty Ltd Sollya heterophylla PO Box 341 KALAMUNDA 6926 December 2012 STATEMENT OF LIMITATIONS Scope of Services This report (“the report”) has been prepared in accordance with the scope of services set out in the contract, or as otherwise agreed, between the Client and Eleanor Bennett (“the Author”). In some circumstances a range of factors such as time, budget, access and/or site disturbance constraints may have limited the scope of services. Reliance on Data In preparing the report, the Author has relied upon data, surveys, analyses, designs, plans and other information provided by the Client and other individuals and organisations, most of which are referred to in the report (“the data”). Except as otherwise stated in the report, the Author has not verified the accuracy or completeness of the data. To the extent that the statements, opinions, facts, information, conclusions and/or recommendations in the report (“conclusions”) are based in whole or part on the data, those conclusions are contingent upon the accuracy and completeness of the data. The Author will not be liable in relation to incorrect conclusions should any data, information or condition be incorrect or have been concealed, withheld, misrepresented or otherwise not fully disclosed to the Author. Environmental Conclusions In accordance with the scope of services, the Author has relied upon the data and has conducted environmental field monitoring and/or testing in the preparation of the report. The nature and extent of monitoring and/or testing conducted is described in the report. -

Native Revegetation Guide Moore River Catchment
Native revegetation guide for the Moore River Catchment Native revegetation guide for the Moore River Catchment Native revegetation guide for the Moore River Catchment A practical guide to native revegetation by soil type in the Moore River catchment Researched and designed by the Moore Catchment Council Funded by the Western Australian Government's State Natural Resource Management Program, supported by Royalties for Regions Native revegetation guide for the Moore River Catchment Welcome Thinking of doing a native revegetation project in the Moore River catchment region but don’t know where to start? This booklet could be for you ! Simple hints and tips to get your native revegetation project off to a flying start. Inside are helpful planning tips, tools and ideas for native species to suit your soil type and location. What are you waiting for……..get planning, get planting ! a Take problem area... Page Content 3 Why revegetate with natives? 4 Planning your revegetation project 6 Moore Catchment soil types 7 Moore Catchment vegetation associations 8 Salmon & York Gum woodland andadvice ...seek help... 9 Wandoo & York Gum woodland 10 Marri & Wandoo woodland 11 Banksia sandplain shrubland & woodland 12 Acacia & York Gum woodland 13 Tamma shrubland ...add ...add some nativeplants... 14 Salt land & creek revegetation 15 Help & Resources 16 Tree nurseries ...equals revegetation...equals success !! 2 Native revegetation guide for the Moore River Catchment Native revegetation guide for the Moore River Catchment Why revegetate with natives? Widespread clearing for agriculture, horticulture and urbanisation has left the Moore’s remnant vegetation vulnerable, fragmented and in some cases critically endangered. East of Moora on the favourable farming soils, only 8-13% remnant vegetation remains. -

Fruits and Seeds of Genera in the Subfamily Faboideae (Fabaceae)
Fruits and Seeds of United States Department of Genera in the Subfamily Agriculture Agricultural Faboideae (Fabaceae) Research Service Technical Bulletin Number 1890 Volume I December 2003 United States Department of Agriculture Fruits and Seeds of Agricultural Research Genera in the Subfamily Service Technical Bulletin Faboideae (Fabaceae) Number 1890 Volume I Joseph H. Kirkbride, Jr., Charles R. Gunn, and Anna L. Weitzman Fruits of A, Centrolobium paraense E.L.R. Tulasne. B, Laburnum anagyroides F.K. Medikus. C, Adesmia boronoides J.D. Hooker. D, Hippocrepis comosa, C. Linnaeus. E, Campylotropis macrocarpa (A.A. von Bunge) A. Rehder. F, Mucuna urens (C. Linnaeus) F.K. Medikus. G, Phaseolus polystachios (C. Linnaeus) N.L. Britton, E.E. Stern, & F. Poggenburg. H, Medicago orbicularis (C. Linnaeus) B. Bartalini. I, Riedeliella graciliflora H.A.T. Harms. J, Medicago arabica (C. Linnaeus) W. Hudson. Kirkbride is a research botanist, U.S. Department of Agriculture, Agricultural Research Service, Systematic Botany and Mycology Laboratory, BARC West Room 304, Building 011A, Beltsville, MD, 20705-2350 (email = [email protected]). Gunn is a botanist (retired) from Brevard, NC (email = [email protected]). Weitzman is a botanist with the Smithsonian Institution, Department of Botany, Washington, DC. Abstract Kirkbride, Joseph H., Jr., Charles R. Gunn, and Anna L radicle junction, Crotalarieae, cuticle, Cytiseae, Weitzman. 2003. Fruits and seeds of genera in the subfamily Dalbergieae, Daleeae, dehiscence, DELTA, Desmodieae, Faboideae (Fabaceae). U. S. Department of Agriculture, Dipteryxeae, distribution, embryo, embryonic axis, en- Technical Bulletin No. 1890, 1,212 pp. docarp, endosperm, epicarp, epicotyl, Euchresteae, Fabeae, fracture line, follicle, funiculus, Galegeae, Genisteae, Technical identification of fruits and seeds of the economi- gynophore, halo, Hedysareae, hilar groove, hilar groove cally important legume plant family (Fabaceae or lips, hilum, Hypocalypteae, hypocotyl, indehiscent, Leguminosae) is often required of U.S. -

Orchid Seed Coat Morphometrics. Molvray and Kores. 1995
American Journal of Botany 82(11): 1443-1454. 1995 . CHARACTER ANALYSIS OF THE SEED COAT IN SPIRANTHOIDEAE AND ORCHIDOIDEAE, WITH SPECIAL REFERENCE TO THE DIURIDEAE (ORCHIDACEAE)I MIA MOLVRAy2 AND PAUL J. KORES Department of Biological Sciences, Loyola University, New Orleans, Louisiana 70118 Previous work on seed types within Orchidaceae has demonstrated that characters associated with the seed coat may have considerable phylogenetic utility. Application of the se characters has been complicated in practice by the absence of quan titative descriptors and in some instances by their apparent lack of congruity with the taxa under con sideration. Using quantitative descriptors of size and shape, we have demonstrated that some of the existing seed classes do not represent well delimited, discrete entities, and we have proposed new seed classes to meet these criteria. In the spiranthoid-orchidoid complex, the characters yielding the most clearly delimited shape classes are cell number and variability and degree and stochasticity of medial cell elongation. Of lesser, but still appreciable, significance are the pre sence of varying types and degrees of intercellular gaps, and some, but not all, features of cell walls. Four seed classes are evident on the basis of these characters in Spiranthoideae and Orchidoideae. These seed types are briefly described, and their distribution among the taxa examined for this study is reported. It is hoped that these more strictly delimited seed classes will faci litate phylogenetic analysis in the family. Phylogenetic relationships within the Orchidaceae delimitation of the seed coat characters within the two have been discussed extensively in a series of recent pub putatively most primitive subfamilies of monandrous or lications by Garay (1960, 1972), Dressler (1981, 1986, chids and evaluates the util ity of these characters for the 1990a, b, c, 1993), Rasmussen (1982, 1986), Burns-Bal purpose of phylogenetic inference, extends this avenue of ogh and Funk (1986), and Chase et aI. -

Redalyc.ARE OUR ORCHIDS SAFE DOWN UNDER?
Lankesteriana International Journal on Orchidology ISSN: 1409-3871 [email protected] Universidad de Costa Rica Costa Rica BACKHOUSE, GARY N. ARE OUR ORCHIDS SAFE DOWN UNDER? A NATIONAL ASSESSMENT OF THREATENED ORCHIDS IN AUSTRALIA Lankesteriana International Journal on Orchidology, vol. 7, núm. 1-2, marzo, 2007, pp. 28- 43 Universidad de Costa Rica Cartago, Costa Rica Available in: http://www.redalyc.org/articulo.oa?id=44339813005 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative LANKESTERIANA 7(1-2): 28-43. 2007. ARE OUR ORCHIDS SAFE DOWN UNDER? A NATIONAL ASSESSMENT OF THREATENED ORCHIDS IN AUSTRALIA GARY N. BACKHOUSE Biodiversity and Ecosystem Services Division, Department of Sustainability and Environment 8 Nicholson Street, East Melbourne, Victoria 3002 Australia [email protected] KEY WORDS:threatened orchids Australia conservation status Introduction Many orchid species are included in this list. This paper examines the listing process for threatened Australia has about 1700 species of orchids, com- orchids in Australia, compares regional and national prising about 1300 named species in about 190 gen- lists of threatened orchids, and provides recommen- era, plus at least 400 undescribed species (Jones dations for improving the process of listing regionally 2006, pers. comm.). About 1400 species (82%) are and nationally threatened orchids. geophytes, almost all deciduous, seasonal species, while 300 species (18%) are evergreen epiphytes Methods and/or lithophytes. At least 95% of this orchid flora is endemic to Australia. -
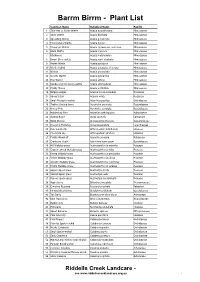
Botanical Name
Barrm Birrm - Plant List Common Name Botanical Name Family 1 Thin-leaf or Snake Wattle Acacia aculeatissima Mimosaceae 2 Silver Wattle Acacia dealbata Mimosaceae 3 Spreading Wattle Acacia genistifolia Mimosaceae 4 Ploughshare Wattle Acacia gunnii Mimosaceae 5 Cinnamon Wattle Acacia leprosa var. uninervia Mimosaceae 6 Black Wattle Acacia mearnsii Mimosaceae 7 Blackwood Acacia melanoxylon Mimosaceae 8 Dwarf Silver-wattle Acacia nano-dealbata Mimosaceae 9 Hedge Wattle Acacia paradoxa Mimosaceae 10 Wattle hybrid Acacia paradoxa x leprosa Mimosaceae 11 Wirilda Acacia provincialis Mimosaceae 12 Golden Wattle Acacia pycnantha Mimosaceae 13 Hop Wattle Acacia stricta Mimosaceae 14 Dandenong Cinnamon-wattle Acacia strictophylla Mimosaceae 15 Prickly Moses Acacia verticillata Mimosaceae 16 Bidgee-widgee Acaena novae-zelandiae Rosaceae 17 Sheep's Burr Acaena ovina Rosaceae 18 Small Mosquito-orchid Acianthus pusillus Orchidaceae 19 Trailing Ground-berry Acrotriche prostrata Epacridaceae 20 Honey Pots Acrotriche serrulata Epacridaceae 21 Maidenhair Fern Adiantum aethiopicum Adiantaceae 22 Austral Bugle Ajuga australis Lamiaceae 23 Black Sheoak Allocasuarina littoralis Casuarinaceae 24 Drooping Mistletoe Amyema pendula Loranthaceae 25 Pale Vanilla-lily Arthropodium milleflorum Liliaceae 26 Chocolate Lily Arthropodium strictum Liliaceae 27 Prickly Woodruff Asperula scoparia Rubiaceae 28 Cranberry Heath Astroloma humifusum Epacridaceae 29 Hill Wallaby-grass Austrodanthonia eriantha Poaceae 30 Copper-awned Wallaby-grass Austrodanthonia fulva Poaceae 31 -

Act Native Woodland Conservation Strategy and Action Plans
ACT NATIVE WOODLAND CONSERVATION STRATEGY AND ACTION PLANS PART A 1 Produced by the Environment, Planning and Sustainable Development © Australian Capital Territory, Canberra 2019 This work is copyright. Apart from any use as permitted under the Copyright Act 1968, no part may be reproduced by any process without written permission from: Director-General, Environment, Planning and Sustainable Development Directorate, ACT Government, GPO Box 158, Canberra ACT 2601. Telephone: 02 6207 1923 Website: www.planning.act.gov.au Acknowledgment to Country We wish to acknowledge the traditional custodians of the land we are meeting on, the Ngunnawal people. We wish to acknowledge and respect their continuing culture and the contribution they make to the life of this city and this region. Accessibility The ACT Government is committed to making its information, services, events and venues as accessible as possible. If you have difficulty reading a standard printed document and would like to receive this publication in an alternative format, such as large print, please phone Access Canberra on 13 22 81 or email the Environment, Planning and Sustainable Development Directorate at [email protected] If English is not your first language and you require a translating and interpreting service, please phone 13 14 50. If you are deaf, or have a speech or hearing impairment, and need the teletypewriter service, please phone 13 36 77 and ask for Access Canberra on 13 22 81. For speak and listen users, please phone 1300 555 727 and ask for Canberra Connect on 13 22 81. For more information on these services visit http://www.relayservice.com.au PRINTED ON RECYCLED PAPER CONTENTS VISION ...........................................................................................................