Arctic Biodiversity Assessment
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
North America Other Continents
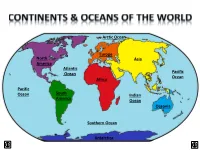
Arctic Ocean Europe North Asia America Atlantic Ocean Pacific Ocean Africa Pacific Ocean South Indian America Ocean Oceania Southern Ocean Antarctica LAND & WATER • The surface of the Earth is covered by approximately 71% water and 29% land. • It contains 7 continents and 5 oceans. Land Water EARTH’S HEMISPHERES • The planet Earth can be divided into four different sections or hemispheres. The Equator is an imaginary horizontal line (latitude) that divides the earth into the Northern and Southern hemispheres, while the Prime Meridian is the imaginary vertical line (longitude) that divides the earth into the Eastern and Western hemispheres. • North America, Earth’s 3rd largest continent, includes 23 countries. It contains Bermuda, Canada, Mexico, the United States of America, all Caribbean and Central America countries, as well as Greenland, which is the world’s largest island. North West East LOCATION South • The continent of North America is located in both the Northern and Western hemispheres. It is surrounded by the Arctic Ocean in the north, by the Atlantic Ocean in the east, and by the Pacific Ocean in the west. • It measures 24,256,000 sq. km and takes up a little more than 16% of the land on Earth. North America 16% Other Continents 84% • North America has an approximate population of almost 529 million people, which is about 8% of the World’s total population. 92% 8% North America Other Continents • The Atlantic Ocean is the second largest of Earth’s Oceans. It covers about 15% of the Earth’s total surface area and approximately 21% of its water surface area. -

Fresh- and Brackish-Water Cold-Tolerant Species of Southern Europe: Migrants from the Paratethys That Colonized the Arctic
water Review Fresh- and Brackish-Water Cold-Tolerant Species of Southern Europe: Migrants from the Paratethys That Colonized the Arctic Valentina S. Artamonova 1, Ivan N. Bolotov 2,3,4, Maxim V. Vinarski 4 and Alexander A. Makhrov 1,4,* 1 A. N. Severtzov Institute of Ecology and Evolution, Russian Academy of Sciences, 119071 Moscow, Russia; [email protected] 2 Laboratory of Molecular Ecology and Phylogenetics, Northern Arctic Federal University, 163002 Arkhangelsk, Russia; [email protected] 3 Federal Center for Integrated Arctic Research, Russian Academy of Sciences, 163000 Arkhangelsk, Russia 4 Laboratory of Macroecology & Biogeography of Invertebrates, Saint Petersburg State University, 199034 Saint Petersburg, Russia; [email protected] * Correspondence: [email protected] Abstract: Analysis of zoogeographic, paleogeographic, and molecular data has shown that the ancestors of many fresh- and brackish-water cold-tolerant hydrobionts of the Mediterranean region and the Danube River basin likely originated in East Asia or Central Asia. The fish genera Gasterosteus, Hucho, Oxynoemacheilus, Salmo, and Schizothorax are examples of these groups among vertebrates, and the genera Magnibursatus (Trematoda), Margaritifera, Potomida, Microcondylaea, Leguminaia, Unio (Mollusca), and Phagocata (Planaria), among invertebrates. There is reason to believe that their ancestors spread to Europe through the Paratethys (or the proto-Paratethys basin that preceded it), where intense speciation took place and new genera of aquatic organisms arose. Some of the forms that originated in the Paratethys colonized the Mediterranean, and overwhelming data indicate that Citation: Artamonova, V.S.; Bolotov, representatives of the genera Salmo, Caspiomyzon, and Ecrobia migrated during the Miocene from I.N.; Vinarski, M.V.; Makhrov, A.A. -

Conservation Planning for Amphibian Species with Complex Habitat Requirements: a Case Study Using Movements and Habitat Selection of the Wood Frog Rana Sylvatica
Journal of Herpetology, Vol. 40, No. 4, pp. 442–453, 2006 Copyright 2006 Society for the Study of Amphibians and Reptiles Conservation Planning for Amphibian Species with Complex Habitat Requirements: A Case Study Using Movements and Habitat Selection of the Wood Frog Rana sylvatica 1,2,3 1,4 2,5 ROBERT F. BALDWIN, ARAM J. K. CALHOUN, AND PHILLIP G. DEMAYNADIER, 1Department of Plant, Soil and Environmental Sciences, University of Maine, Orono, Maine 04469, USA 2Maine Department of Inland Fisheries and Wildlife, 650 State Street, Bangor, Maine 04401, USA ABSTRACT.—Conservation of fauna breeding in vernal pools is challenging given their complex life histories. Many species, including the widespread North American Wood Frog (Rana sylvatica), require both aquatic and terrestrial habitat, yet insufficient information exists about movements between these environments, nor fine-scale selection patterns within them. To inform conservation planning, we conducted a radio-telemetry study of seasonal patterns of Wood Frog movements and habitat selection in southern Maine. Forty-three frogs were tracked an average of 25.6 days each, April to November 2003. In early spring, Wood Frogs generally selected damp leaf litter retreats on the margins of breeding pools. Following breeding, frogs selected forested wetlands (9.3% of the landscape) over forested uplands (90.7% of the landscape) in 75.3% of radio locations (N 5 544). Postbreeding movements from breeding pools to nearby, closed-canopy, forested wetlands ranged from 102–340 m (median 169m, N 5 8) and included stopovers in upland forest floors ranging from one to 17 days (median two days, N 5 7). -

Red-Legged Frog Rana Aurora
COSEWIC Assessment and Update Status Report on the Red-legged Frog Rana aurora in Canada SPECIAL CONCERN 2004 COSEWIC COSEPAC COMMITTEE ON THE STATUS OF COMITÉ SUR LA SITUATION ENDANGERED WILDLIFE DES ESPÈCES EN PÉRIL IN CANADA AU CANADA COSEWIC status reports are working documents used in assigning the status of wildlife species suspected of being at risk. This report may be cited as follows: COSEWIC 2004. COSEWIC assessment and update status report on the Red-legged Frog Rana aurora in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. vi + 46 pp. (www.sararegistry.gc.ca/status/status_e.cfm). Previous report: Waye, H. 1999. COSEWIC status report on the red-legged frog Rana aurora in Canada in COSEWIC assessment and status report on the red-legged frog Rana aurora in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. 1-31 pp. Production note: COSEWIC would like to acknowledge Kristiina Ovaska and Lennart Sopuck for writing the status report on the Red-legged Frog Rana aurora. This report was prepared under contract with Environment Canada and was overseen and edited by David Green, the COSEWIC Amphibians and Reptiles Species Specialist Subcommittee Co-chair. For additional copies contact: COSEWIC Secretariat c/o Canadian Wildlife Service Environment Canada Ottawa, ON K1A 0H3 Tel.: (819) 997-4991 / (819) 953-3215 Fax: (819) 994-3684 E-mail: COSEWIC/[email protected] http://www.cosewic.gc.ca Ếgalement disponible en français sous le titre Ếvaluation et Rapport de situation du COSEPAC sur la situation de Grenouille à pattes rouges (Rana aurora) au Canada — Mise à jour. -

Spotted Chorus Frog
This Article From Reptile & Amphibian Profiles From February, 2005 The Cross Timbers Herpetologist Newsletter of the Dallas-Fort Worth Herpetological Society Dallas-Fort Worth Herpetological Society is a 501(c)3 nonprofit organiza- tion whose mission is: To promote understanding, appreciation, and conservation Spotted Chorus Frog ( Pseudacris clarkii ) of reptiles and amphibians, to encourage respect for their By Michael Smith habitats, and to foster respon- Texas winters can be cold, with biting loud for a frog of such small size. As the frogs sible captive care. winds and the occasional ice storm. But Texas brace against prairie grasses in the shallow wa- winters are often like the tiny north Texas ter, the throats of the males expand into an air- towns of Santo or Venus – you see them com- filled sac and call “wrret…wrret…wrret” to All articles and photos remain ing down the road, but if you blink twice nearby females. under the copyright of the au- you’ve missed them. And so, by February we Spotted chorus frogs are among the small thor and photographer. This often have sunny days where a naturalist’s and easily overlooked herps of our area, but publication may be redistributed thoughts turn to springtime. Those earliest they are beautiful animals with interesting life- in its original form, but to use the sunny days remind us that life and color will article or photos, please contact: return to the fields and woods. styles. [email protected] In late February or early March, spring Classification rains begin, and water collects in low places. -

Wood Frog (Rana Sylvatica): a Technical Conservation Assessment
Wood Frog (Rana sylvatica): A Technical Conservation Assessment Prepared for the USDA Forest Service, Rocky Mountain Region, Species Conservation Project March 24, 2005 Erin Muths1, Suzanne Rittmann1, Jason Irwin2, Doug Keinath3, Rick Scherer4 1 U.S. Geological Survey, Fort Collins Science Center, 2150 Centre Ave. Bldg C, Fort Collins, CO 80526 2 Department of Biology, Bucknell University, Lewisburg, PA 17837 3 Wyoming Natural Diversity Database, University of Wyoming, P.O. Box 3381, Laramie, WY 82072 4 Colorado State University, GDPE, Fort Collins, CO 80524 Peer Review Administered by Society for Conservation Biology Muths, E., S. Rittman, J. Irwin, D. Keinath, and R. Scherer. (2005, March 24). Wood Frog (Rana sylvatica): a technical conservation assessment. [Online]. USDA Forest Service, Rocky Mountain Region. Available: http://www.fs.fed.us/r2/projects/scp/assessments/woodfrog.pdf [date of access]. ACKNOWLEDGMENTS The authors would like to acknowledge the help of the many people who contributed time and answered questions during our review of the literature. AUTHORS’ BIOGRAPHIES Dr. Erin Muths is a Zoologist with the U.S. Geological Survey – Fort Collins Science Center. She has been studying amphibians in Colorado and the Rocky Mountain Region for the last 10 years. Her research focuses on demographics of boreal toads, wood frogs and chorus frogs and methods research. She is a principle investigator for the USDOI Amphibian Research and Monitoring Initiative and is an Associate Editor for the Northwestern Naturalist. Dr. Muths earned a B.S. in Wildlife Ecology from the University of Wisconsin, Madison (1986); a M.S. in Biology (Systematics and Ecology) from Kansas State University (1990) and a Ph.D. -

Geography Notes.Pdf
THE GLOBE What is a globe? a small model of the Earth Parts of a globe: equator - the line on the globe halfway between the North Pole and the South Pole poles - the northern-most and southern-most points on the Earth 1. North Pole 2. South Pole hemispheres - half of the earth, divided by the equator (North & South) and the prime meridian (East and West) 1. Northern Hemisphere 2. Southern Hemisphere 3. Eastern Hemisphere 4. Western Hemisphere continents - the largest land areas on Earth 1. North America 2. South America 3. Europe 4. Asia 5. Africa 6. Australia 7. Antarctica oceans - the largest water areas on Earth 1. Atlantic Ocean 2. Pacific Ocean 3. Indian Ocean 4. Arctic Ocean 5. Antarctic Ocean WORLD MAP ** NOTE: Our textbooks call the “Southern Ocean” the “Antarctic Ocean” ** North America The three major countries of North America are: 1. Canada 2. United States 3. Mexico Where Do We Live? We live in the Western & Northern Hemispheres. We live on the continent of North America. The other 2 large countries on this continent are Canada and Mexico. The name of our country is the United States. There are 50 states in it, but when it first became a country, there were only 13 states. The name of our state is New York. Its capital city is Albany. GEOGRAPHY STUDY GUIDE You will need to know: VOCABULARY: equator globe hemisphere continent ocean compass WORLD MAP - be able to label 7 continents and 5 oceans 3 Large Countries of North America 1. United States 2. Canada 3. -

Rana Temporaria)
European Community Directive on the Conservation of Natural Habitats and of Wild Fauna and Flora (92/43/EEC) Fourth Report by the United Kingdom under Article 17 on the implementation of the Directive from January 2013 to December 2018 Supporting documentation for the conservation status assessment for the species: S1213 ‐ Common frog (Rana temporaria) ENGLAND IMPORTANT NOTE ‐ PLEASE READ • The information in this document is a country‐level contribution to the UK Reporton the conservation status of this species, submitted to the European Commission aspart of the 2019 UK Reporting under Article 17 of the EU Habitats Directive. • The 2019 Article 17 UK Approach document provides details on how this supporting information was used to produce the UK Report. • The UK Report on the conservation status of this species is provided in a separate doc‐ ument. • The reporting fields and options used are aligned to those set out in the European Com‐ mission guidance. • Explanatory notes (where provided) by the country are included at the end. These pro‐ vide an audit trail of relevant supporting information. • Some of the reporting fields have been left blank because either: (i) there was insuffi‐ cient information to complete the field; (ii) completion of the field was not obligatory; (iii) the field was not relevant to this species (section 12 Natura 2000 coverage forAnnex II species) and/or (iv) the field was only relevant at UK‐level (sections 9 Future prospects and 10 Conclusions). • For technical reasons, the country‐level future trends for Range, Population and Habitat for the species are only available in a separate spreadsheet that contains all the country‐ level supporting information. -

Manchuria Documents to Examine
Source 1 Source Information: The Legacy of the Soviet Union Offensives of August 1945 https://amti.csis.org/the-legacy-of-the-soviet-offensives-of-august-1945/ ____________________________________________________________________________ THE LEGACY OF THE SOVIET OFFENSIVES OF AUGUST 1945 BY JEFF MANKOFF | AUGUST 13, 2015 JAPAN, RUSSIA, UNITED STATES The Second World War was an unparalleled calamity for the Soviet Union. As many as 27 million Soviet soldiers and civilians died as a result of the conflict that started with the German invasion of Poland in September 1939 and ended with the Japanese surrender in August 1945. Consumed by this existential struggle along its western border, the Soviet Union was a comparatively minor factor in the Pacific War until the very end. Yet Moscow’s timely intervention in the war against Japan allowed it to expand its influence along the Pacific Rim. With the breakdown of Allied unity soon heralding the onset of the Cold War, Soviet gains in Asia also left a legacy of division and confrontation, some of which endure into the present. By the 1930s, Stalin’s Soviet Union and Imperial Japan both viewed themselves as rising powers with ambitions to extend their territorial holdings. In addition to a strategic rivalry dating back to the 19th century, they now nursed an ideological enmity born of the Bolshevik Revolution and the ultraconservative military’s growing hold on Japanese politics. In 1935, Japan signed the AntiComintern Pact with Hitler’s Germany, laying the foundation for the creation of the Axis (Fascist Italy would join the following year). The two militaries engaged in a series of skirmishes along the frontier between Soviet Siberia and Japanese-occupied Manchuria (Manchukuo) during the late 1930s. -

Botanical Problems in Boreal America. I Author(S): Hugh M
Botanical Problems in Boreal America. I Author(s): Hugh M. Raup Source: Botanical Review, Vol. 7, No. 3, Botanical Problems in Boreal America. I (Mar., 1941), pp. 147-208 Published by: Springer on behalf of New York Botanical Garden Press Stable URL: http://www.jstor.org/stable/4353246 Accessed: 15-12-2017 21:12 UTC JSTOR is a not-for-profit service that helps scholars, researchers, and students discover, use, and build upon a wide range of content in a trusted digital archive. We use information technology and tools to increase productivity and facilitate new forms of scholarship. For more information about JSTOR, please contact [email protected]. Your use of the JSTOR archive indicates your acceptance of the Terms & Conditions of Use, available at http://about.jstor.org/terms Springer, New York Botanical Garden Press are collaborating with JSTOR to digitize, preserve and extend access to Botanical Review This content downloaded from 128.103.149.52 on Fri, 15 Dec 2017 21:12:58 UTC All use subject to http://about.jstor.org/terms THE BOTANICAL REVIEW VOL. VII MARCH, 1941 No. 3 BOTANICAL PROBLEMS IN BOREAL AMERICA. I HUGH M. RAUP Arnold Arboretum, Harvard University CONTENTS PAGE Introduction ................ ........................... 148 Acknowledgments . ........................................... 150 Exploration .............. ............................. 151 Physiographic History ........................................... 161 Climate ........................................................... 169 Origin and Distribution of the Flora Speciation and Endemism .173 The Theory of Persistence The Darwin-Hooker Concept ............................... 178 The Nunatak Hypothesis .......... .......... 181 Conservatism vs. Aggressiveness ............................. 184 Wynne-Edwards' Criticism of the Nunatak Hypothesis . 186 Discussion of Wynne-Edwards' Criticism .188 Hulten's Studies of Arctic and Boreal Biota Statement of the Problem .198 Plastic vs. -

Parasitic Nematodes of Pool Frog (Pelophylax Lessonae) in the Volga Basin
Journal MVZ Cordoba 2019; 24(3):7314-7321. https://doi.org/10.21897/rmvz.1501 Research article Parasitic nematodes of Pool Frog (Pelophylax lessonae) in the Volga Basin Igor V. Chikhlyaev1 ; Alexander B. Ruchin2* ; Alexander I. Fayzulin1 1Institute of Ecology of the Volga River Basin, Russian Academy of Sciences, Togliatti, Russia 2Mordovia State Nature Reserve and National Park «Smolny», Saransk, Russia. *Correspondence: [email protected] Received: Febrary 2019; Accepted: July 2019; Published: August 2019. ABSTRACT Objetive. Present a modern review of the nematodes fauna of the pool frog Pelophylax lessonae (Camerano, 1882) from Volga basin populations on the basis of our own research and literature sources analysis. Materials and methods. Present work consolidates data from different helminthological works over the past 80 years, supported by our own research results. During the period from 1936 to 2016 different authors examined 1460 specimens of pool frog, using the method of full helminthological autopsy, from 13 regions of the Volga basin. Results. In total 9 nematodes species were recorded. Nematode Icosiella neglecta found for the first time in the studied host from the territory of Russia and Volga basin. Three species appeared to be more widespread: Oswaldocruzia filiformis, Cosmocerca ornata and Icosiella neglecta. For each helminth species the following information included: systematic position, areas of detection, localization, biology, list of definitive hosts, the level of host-specificity. Conclusions. Nematodes of pool frog, excluding I. neglecta, belong to the group of soil-transmitted helminthes (geohelminth) and parasitize in adult stages. Some species (O. filiformis, C. ornata, I. neglecta) are widespread in the host range. -

THE AGILE FROG Species Action Plan Rana Dalmatina SUMMARSUMMARSUMMARYYY DOCUMENT
THE AGILE FROG Species Action Plan Rana dalmatina SUMMARSUMMARSUMMARYYY DOCUMENT The agile frog Rana dalmatina is Despite the efforts of these distributed widely throughout much of organisations, the future of Jersey’s southern and central Europe, but is agile frog is still far from secure. The found in only a few northern locations factors which probably played a key including Jersey - the frog is not found role in the frogs decline are still very anywhere else in the British Isles. The much in evidence: Jersey population of the agile frog has been declining in both range and •water quality and quantity, as a result SSSpppecial pointsss ofofof numbers since the early 1900’s. In the of intensive agriculture, are still below inininteresteresteresttt::: 1970’s only seven localities were listed EU standards in many areas; The agile frog is protected where the frog could still be found, and •the continuing alteration, disturbance, under schedule 1 of the by the mid 1980’s this had fallen to and loss of potentially suitable Conservation of Wildlife only two sites. In 1987 one of the amphibian habitat; (Jersey) Law 2000. remaining two populations was lost as a •the growing numbers of predatory result of a lethal spill of agricultural ducks, cats, and feral ferrets. DESCRIPTION pesticide into the breeding pond. The The agile frog Rana dalmatina species is now believed to be confined These and other factors have combined is a European brown frog, to a single vulnerable population in the to reduce the frog population to the growing up to 90 mm (snout south-west of the island.