Protonibea Diacanthus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Optimising the Management of Tropical Reef Fish Through the Development of Indigenous Scientific Capability
DEPARTMENT OF PRIMARY INDUSTRY AND RESOURCES Optimising the Management of Tropical Reef Fish through the Development of Indigenous Scientific Capability FRDC Project No. 2013/17 www.nt.gov.au FRDC Project No. 2013/17 Optimising the Management of Tropical Reef Fish through the Development of Indigenous Scientific Capability Thor Saunders, Diane P. Barton, David Crook, Jenny Ovenden, Stephen J. Newman, Richard Saunders, Laura Taillebois, Jonathan Taylor, Michael J. Travers, Christine Dudgeon, Safia Maher and David J. Welch May 2017 FRDC Project No 2013/017 Fishery Report No. 117 ISBN: 978-0-7245-4769-2 DPIR Fishery Report No. 117 Page i FRDC Project No. 2013/17 Copyright ©: Fisheries Research and Development Corporation and the Northern Territory Government, 2017 This work is copyright. Except as permitted under the Copyright Act 1968 (Commonwealth), no part of this publication may be reproduced by any process, electronic or otherwise, without the specific written permission of the copyright owners. Neither may information be stored electronically in any form whatsoever without such permission. Ownership of Intellectual property rights Unless otherwise noted, copyright (and any other intellectual property rights, if any) in this publication is owned by the Fisheries Research and Development Corporation and the Northern Territory Government. This publication (and any information sourced from it) should be attributed to Saunders, T., Barton, D., Crook, D., Ovenden, J., Newman, S.J., Saunders, R., Taillebois, L., Taylor, J., Travers, M.J., Dudgeon, C., Maher, S. and Welch, D.J. (2016), Optimising the management of tropical reef fish through the development of Indigenous scientific capability. Darwin, Northern Territory, Fishery Report No. -

House of Representatives
COMMONWEALTH OF AUSTRALIA Official Committee Hansard HOUSE OF REPRESENTATIVES STANDING COMMITTEE ON ABORIGINAL AND TORRES STRAIT ISLANDER AFFAIRS Reference: Capacity building in Indigenous communities WEDNESDAY, 27 NOVEMBER 2002 DARWIN BY AUTHORITY OF THE HOUSE OF REPRESENTATIVES INTERNET The Proof and Official Hansard transcripts of Senate committee hearings, some House of Representatives committee hearings and some joint com- mittee hearings are available on the Internet. Some House of Representa- tives committees and some joint committees make available only Official Hansard transcripts. The Internet address is: http://www.aph.gov.au/hansard To search the parliamentary database, go to: http://search.aph.gov.au HOUSE OF REPRESENTATIVES STANDING COMMITTEE ON ABORIGINAL AND TORRES STRAIT ISLANDER AFFAIRS Wednesday, 27 November 2002 Members: Mr Wakelin (Chair), Mr Danby, Mrs Draper, Mr Haase, Ms Hoare, Mrs Hull, Dr Lawrence, Mr Lloyd, Mr Snowdon and Mr Tollner. Members in attendance: Ms Hoare, Mr Lloyd, Mr Snowdon, Mr Tollner and Mr Wakelin. Terms of reference for the inquiry: To inquire into and report on: Strategies to assist Aboriginals and Torres Strait Islanders better manage the delivery of services within their communities. In particular, the committee will consider building the capacities of: (a) community members to better support families, community organisations and representative councils so as to deliver the best outcomes for individuals, families and communities; (b) Indigenous organisations to better deliver and influence -

Northern Territory) Act 1976
Aboriginal Land Rights (Northern Territory) Act 1976 No. 191, 1976 Compilation No. 41 Compilation date: 4 April 2019 Includes amendments up to: Act No. 27, 2019 Registered: 15 April 2019 Prepared by the Office of Parliamentary Counsel, Canberra Authorised Version C2019C00143 registered 15/04/2019 About this compilation This compilation This is a compilation of the Aboriginal Land Rights (Northern Territory) Act 1976 that shows the text of the law as amended and in force on 4 April 2019 (the compilation date). The notes at the end of this compilation (the endnotes) include information about amending laws and the amendment history of provisions of the compiled law. Uncommenced amendments The effect of uncommenced amendments is not shown in the text of the compiled law. Any uncommenced amendments affecting the law are accessible on the Legislation Register (www.legislation.gov.au). The details of amendments made up to, but not commenced at, the compilation date are underlined in the endnotes. For more information on any uncommenced amendments, see the series page on the Legislation Register for the compiled law. Application, saving and transitional provisions for provisions and amendments If the operation of a provision or amendment of the compiled law is affected by an application, saving or transitional provision that is not included in this compilation, details are included in the endnotes. Editorial changes For more information about any editorial changes made in this compilation, see the endnotes. Modifications If the compiled law is modified by another law, the compiled law operates as modified but the modification does not amend the text of the law. -

Fishery Status Reports 2010
Fishery Report No. 106 November 2011 Northern Territory Government Department of Resources GPO Box 3000 Darwin NT 0801 AUSTRALIA © Copyright Northern Territory Government 2011 This work is copyright. Except as permitted under the Copyright Act 1968 (Commonwealth) no part of this publication may be reproduced by any process, electronic or otherwise, without the specific written permission of the copyright owners. Nor may information be stored electronically in any form whatsoever without such permission. Disclaimer While all care has been taken to ensure that information contained in the Fishery Status Reports is true and correct at the time of publication, changes in circumstances after the time of publication may impact on the accuracy of its information. The Northern Territory of Australia gives no warranty or assurance, and makes no representation as to the accuracy of any information or advice contained in this Fishery Report, or that it is suitable for your intended use. You should not rely upon information in this publication for the purpose of making any serious, business or investment decisions without obtaining independent and/or professional advice in relation to your particular situation. The Northern Territory of Australia disclaims any liability or responsibility or duty of care towards any person for loss or damage caused by any use of or reliance on the information contained in this publication. November 2011 Bibliography Northern Territory Government (2011). Fishery Status Reports 2009. Northern Territory Government Department of Resources. Fishery Report No. 106. Fishery Report No. 106 ISSN 1832-7818 Page ii Contents INTRODUCTION .............................................................................................................................1 NT FISHERIES – 2010 HIGHLIGHTS AND 2011 PRIORITIES ...................................................... -

Magnetic Map of the Northern Territory, 1:2 500 000 Scale
MAGNETIC MAP of the NORTHERN TERRITORY AIRBORNE SURVEY SPECIFICATIONS MELVILLE ISLAND CAPE DON ARAFURA SEA WESSEL ISLANDS TIMOR SEA BATHURST VAN DIEMEN GULF ISLAND BEAGLE 132°E 135°E GULF 129°E 138°E GULF OF JUNCTION BAY MELVILLE ISLAND COBOURG PENINSULA WESSEL ISLANDS TRUANT ISLAND BATHURST ISLAND PERON ISLANDS CARPENTARIA JOSEPH MELVILLE BONAPARTE GROOTE ISLAND EYLAN CAPE GULF DT DON ARAFURA SEA WESSEL ISLANDS TIMOR MARIA ISLAND SIR EDWARD PELLEW GROUP SEA Airborne Survey BATHURST VAN DIEMEN GULF ISLAND Flight Line Spacing (m) MILINGIMBI 12°S DARWIN ALLIGATOR RIVER ARNHEM BAY GOVE 12°S FOG BAY BEAGLE 150 GULF DARWIN GULF 200 250 OF 300 400 MOUNT MARUMBA PINE CREEK MOUNT EVELYN BLUE MUD BAY PORT LANGDON CAPE SCOTT 500 PERON ISLANDS CARPENTARIA > 500 JOSEPH BONAPARTE GROOTE GULF EYLANDT URAPUNGA FERGUSSON RIVER KATHERINE ROPER RIVER CAPE BEATRICE The quality of the data used to PORT KEATS construct the magnetic image is a function of the flight line spacing, along line sampling, flying height and positional accuracy. KATHERINE The predominant regional-scale line spacing of magnetic data included in the Magnetic Map of the Northern Territory is shown here. Some smaller high MARIA ISLAND resolution surveys are included in HODGSON DOWNS this merge; these are not 15°S DELAMERE LARRIMAH MOUNT YOUNG PELLEW 15°S AUVERGNE displayed. 0 100 200 300 400 500 km SIR EDWARD PELLEW GROUP GEOLOGICAL REGIONS TANUMBIRINI VICTORIA RIVER DOWNS DALY WATERS BAUHINIA DOWNS ROBINSON RIVER WATERLOO Mesozoic-Cenozoic Neoproterozoic- Palaeozoic BEETALOO WAVE HILL NEWCASTLE WATERS WALHALLOW CALVERT HILLS Palaeo-Mesoproterozoic LIMBUNYA Basins Palaeo-Mesoproterozoic Orogens Archaean The Northern Territory is dominated by Palaeo- to HELEN SPRINGS 18°S WINNECKE CREEK SOUTH LAKE WOODS BRUNETTE DOWNS MOUNT DRUMMOND 18°S Mesoproterozoic orogens and BIRRINDUDU basins, with localised outcrop of Neoarchean (2.7-2.5 Ga) granite and gneiss. -

List of Northern Territory Birds
WESTERN AUSTRALIAN MUSEUM SPECIAL PUBLICA TIO N No. 4 LIST OF NORTHERN TERRITORY BIRDS World List Abbreviation : Spec. Publ. W. Aust. Mus. Printed for the Western Australian Museum Board by the Government Printer, Perth, Western Austral,ia. Iss~ecl 28th February, 1967 Edited by w. D. L. RIDE and A. NEUMANN RED- WINGED PARROT Photograph by Mr. Peter Slater LIST OF NORTHERN TERRITORY BIRDS BY G. M. STORR [2]-13314 Table of Contents INTRODUCTION 7 Classification ... 7 Distribution ... 8 Status, habitat and breeding season 8 Appendices 9 LIST OF BIRDS 11 BIBLIOGRAPHY 65 SPECIES CONFIRMANDAE .... 69 GAZETTEER .... 71 INDEX TO SPECIES .. 84 Introduction It has long been the desire of ornithologists to have a list of Australian birds with their known range set out with considerably more precision than in current checklists. Yet it is hard to see how such a list can be compiled until each of the states and territories has a list of its own. Several state lists have appeared in the last two decades, and the only large gaps remaining are the birds of Queensland and the Northern Territory. In choosing the second as my subject, I have undertaken much the lighter task; for the avi fauna of the Territory is impoverished compared to Queensland's, and its literature is far smaller. The present work is a compendium of what has been published on the occurrence, status, habitat and breeding season of Northern Territory birds, augmented with my own field notes and those of Dr. D. L. Serventy. No attempt has been made to fill the numerous gaps in the record by writing to museums and observers for information or by personally exploring un worked regions. -

Interim Marine and Coastal Regionalisation for Australia
Interim Marine and Coastal Regionalisation for Australia: An ecosystem-based classification for marine and coastal environments IMCRA Technical Group June 1998 • Version 3.3 The regionalisations presented in this report were compiled from regional frameworks developed by the Commonwealth, States and Northern Territory marine management and research agencies. The compilation was coordinated by the Biodiversity Group, Environment Australia. IMCRA Version 3.3 was developed through the technical input, information and advice of the following agencies and individuals. Numerous people contributed to previous versions of IMCRA and are acknowledged in the Acknowledgments. IMCRA Technical Group Commonwealth Australian Geological Survey Organisation (Bob Burne, now with the Australian National University) Biodiversity Group, Environment Australia (Ian Cresswell and Richard Thackway) CSIRO Division of Marine Research (Vincent Lyne and Peter Last) CSIRO Division of Wildlife and Ecology (Neil Hamilton) Environmental Resource Information Network (ERIN), Environment Australia (Steve Blake) Great Barrier Reef Marine Park Authority (Jim Muldoon and Joan Phillips) New South Wales New South Wales Fisheries (David Pollard) New South Wales National Parks and Wildlife Service (Ian Brown) Northern Territory Parks and Wildlife Commission (Ron Billyard) Queensland Department of Environment (Tim Stevens) South Australia South Australian Research and Development Institute (Karen Edyvane) Department of Environment and Natural Resources (Doug Fotheringham) Tasmania Department of Environment and Land Management (Peter Bosworth and Dave Peters) University of Tasmania (Graham Edgar) Victoria Department of Natural Resources and Environment (Don Hough and Chris Ashe) Western Australia Department of Conservation and Land Management (Chris Simpson) The report has been prepared under the auspices of the ANZECC Task Force on Marine Protected Areas. -
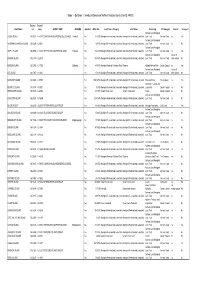
Stage 1 - Database 1: Introduced Species on Northern Territory Islands (Com ID: 49530)
Stage 1 - Database 1: Introduced Species on Northern Territory Islands (Com ID: 49530) Decimal Decimal Island Name Lat Long GROUP_NAME INDNAME Gazetteer AREA_HA_ Land Tenure Category Land Status Ownership NT Bioregion Joined? Surveyed? Arnhem Land Aboriginal ALGER ISLAND 135.96611 -11.87810 CUNNINGHAM ISLANDS/WESSEL ISLANDS Toombuli Yes 845.333 Aboriginal Freehold and Leasehold Aboriginal Freehold and Leasehold Land Trust Arnhem Coast no Yes Arnhem Land Aboriginal ANGARMBULUMARDJA ISLAND 136.63495 -13.66611 Yes 169.956 Aboriginal Freehold and Leasehold Aboriginal Freehold and Leasehold Land Trust Arnhem Coast no No Arnhem Land Aboriginal ASTELL ISLAND 136.39856 -11.88248 THE ENGLISH COMPANYS ISLANDS Yinbadak Yes 1264.439 Aboriginal Freehold and Leasehold Aboriginal Freehold and Leasehold Land Trust Arnhem Coast no Yes Arnhem Land Aboriginal not an off BANYAN ISLAND 135.21349 -12.25819 Yes 4943.473 Aboriginal Freehold and Leasehold Aboriginal Freehold and Leasehold Land Trust Arnhem Coast shore island No BARRON ISLAND 132.35042 -12.17392 Djidbordu Yes 450.472 Aboriginal Freehold - National Park Reserve Kakadu National Park Darwin Coastal no Yes Arnhem Land Aboriginal not an off BAT ISLAND 134.17367 -12.10151 Yes 376.139 Aboriginal Freehold and Leasehold Aboriginal Freehold and Leasehold Land Trust Arnhem Coast shore island No BATHURST ISLAND 130.32948 -11.57578 Yes 169319.353 Aboriginal Freehold and Leasehold Aboriginal Freehold and Leasehold Tiwi Land Trust Tiwi Cobourg no Yes Aboriginal Freehold and BEATRICE ISLAND 135.78374 -15.09723 -

••• Australian Government •X• Northern Territory Government
The natural occurrence of northern quolls Dasyurus hal/ucatus on islands of the Northern Territory: assessment of refuges from the threat posed by cane toads Bufo marinus. John Woinarski, Brooke Rankmore, Alaric Fisher, Kym Brennan and Damian Milne Report to The Australian Government's Natural Heritage Trust December 2007 ••• Australian Government •X• Northern Territory Government .J Picture above and front cover: Northern quell on Jc:gged Head Island, off Groote Eylandt (photo Mani Berghout) Picture right: Part of the Bromby islands chain, off north-eastern Arnhem Land. (photo: Kym Brennan) 11 Summary Recently, the northern quell Dasyurus ha/lucatus has suffered widespread and rapid decline across its mainland range because of its susceptibility to poisoning by the invading cane toad Bufo marinus. Island populations may now offer the greatest long-term security for the northern quell. Here, we collate all available information on the occurrence of northern quells on Northern Territory islands, and report on two surveys to previously-unsampled sets of islands. We list 233 islands >20 ha in the Northern Territory. Northern quells are known to occur naturally on 10 of these islands, known to be absent from 58 islands, and probably absent (on the basis of lack of reports from inhabited or visited islands) from a further 35 islands. This set of islands is an unrepresentative sample of the total island pool, with larger more rugged islands more likely to have been sampled. The pattern of occurrence of quells on Northern Territory islands was examined with generalised linear modelling, with one model including only definite present and absent records, and the other model also including probable absences. -

GEOLOGICAL MAP of the NORTHERN TERRITORY TERTIARY Cz Fluvial Sandstone and Siltstone on Bathurst and Melville Islands
Ma Sand, silt and clay in coastal esturies 0 QUARTERNARY Qa Sand, clay, calcrete and lucastrine limestone in inland palaeodrainage; GEOLOGICAL MAP of the NORTHERN TERRITORY TERTIARY Cz fluvial sandstone and siltstone on Bathurst and Melville Islands 70 MONEY SHOAL, BONAPARTE, ARAFURA AND PEDIRKA AND EROMANGA 132°E 135°E CARPENTARIA BASINS BASINS 129°E 138°E Mudstone, Shale, shale sandstone K K CRETACEOUS 100 JUNCTION BAY MELVILLE ISLAND COBOURG PENINSULA WESSEL ISLANDS TRUANT ISLAND BATHURST ISLAND Minjilang Sandstone, Shale, shale, * Units not exposed sandstone MELVILLE * Jk mudstone * Jk ISLAND Johnston CAPE DON WESSEL ISLANDS JURASSIC Sandstone, Sandstone, Milikapiti Qa shale, shale Qa ARAFURA SEA * J coal * J Pularumpi Tjipripu River Murenella 200 TIMOR K Sandstone, Sandstone, River K Qa TRIASSIC shale, shale, Cz Warruwi * T limestone * T coal Cz Paru Pickertaramoor M10 Nguiu Qa Creek K SEA K Sandstone, Sandstone, Murgenella River PERMIAN limestone, shale, BATHURST VAN DIEMEN GULF P shale, coal, AMADEUS, NGALIA, DALY, P coal ISLAND King Galiwinku diamictite GEORGINA AND WISO BASINS Sandstone, Sandstone, 300 ALLIGATOR RIVER Coopers MILINGIMBI K Qa ARNHEM BAY 12°S DARWIN g4 M10 GOVE 12°S conglomerate, conglomerate, FOG BAY K K Maningrida siltstone, Pn Creek Milingimbi d9 M9 M8 CARBONIFEROUS siltstone, g4 C shale ALICE SPRINGS OROGENY 400-300 BEAGLE * DC shale, ADELAIDE K Qa M10 Qa M6 Nhulunbuy coal, Qa d6 Qa Qa Cato Yirrkala diamictite GULF Qa River g4 SOUTH ALLIGATOR Woolen g6 Sandstone, Qa Oenpelli River K f6 g5 DARWIN Wildman Ramingining -
The Natural Occurrence of Northern Quolls Dasyurus Hallucatus On
The natural occurrence of northern quolls Dasyurus hallucatus on islands of the Northern Territory: assessment of refuges from the threat posed by cane toads Bufo marinus. John Woinarski, Brooke Rankmore, Alaric Fisher, Kym Brennan and Damian Milne Report to The Australian Government’s Natural Heritage Trust December 2007 Picture above and front cover: Northern quoll on Jagged Head Island, off Groote Eylandt (photo Mani Berghout) Picture right: Part of the Bromby islands chain, off north-eastern Arnhem Land. (photo: Kym Brennan) ii Summary Recently, the northern quoll Dasyurus hallucatus has suffered widespread and rapid decline across its mainland range because of its susceptibility to poisoning by the invading cane toad Bufo marinus. Island populations may now offer the greatest long-term security for the northern quoll. Here, we collate all available information on the occurrence of northern quolls on Northern Territory islands, and report on two surveys to previously-unsampled sets of islands. We list 233 islands >20 ha in the Northern Territory. Northern quolls are known to occur naturally on 10 of these islands, known to be absent from 58 islands, and probably absent (on the basis of lack of reports from inhabited or visited islands) from a further 35 islands. This set of islands is an unrepresentative sample of the total island pool, with larger more rugged islands more likely to have been sampled. The pattern of occurrence of quolls on Northern Territory islands was examined with generalised linear modelling, with one model including only definite present and absent records, and the other model also including probable absences. -
Birds of the Northern Territory
WESTERN AUSTRALIAN MUSEUM SPECIAL PUBLICATION No. 7 Birds of tile I erritory Western Australian Museum Special Publication No. 7 Birds of the Northern Territory by G.M. Starr Perth 1977 EDITOR: A.F. LOVELL World List Abbreviation: Spec. PubIs West. Aust. Mus. ISBN 0 7244 6281 3 ISSN 0083-873X Printed and published at the Western Australian Museum, Frands Street, Perth. 284411. 4 TABLE OF CONTENTS Page Introduction 7 List of Birds 8 Gazetteer ". 105 Index 115 5 ---------------------------- ----~--- INTRODUCTION Ornithology in the Northern Territory has proceeded by fits and starts. The pioneering work of Gilbert, Stokes and Bynoe in 1839-41 was followed by many decades in which little or nothing was added to our knowledge of northern birds. Then came the great period 1894-1916, when Keartland, Dahl, Le Souef, Tunney, Hill, Barnard, Rogers and .McLennan laid the foundations of Northern Territory ornithology. The next half century was one of quiet consolidation. In 1967 I brought out a List of Northern Territory Birds (Spec. PubIs West. Aust. Mus. No. 4) which summarised our knowledge of distribution, relative abundance, habitat preferences, movements and breeding season. That paper was quickly rendered obsolete by a decade of intensive field work, notably by the Harold Hall Expedition to Arnhem Land etc., S.A. Parker and associates in Central Australia, D.N. Crawford and other CSIRO personnel in the far north, Julian Ford and colleagues in the far southwest, and W.H. Butler (on behalf of. the American Museum of Natural History) on Ellery Creek, the Roper and the Daly, and in the Pinkerton Range.