Realisation of the Study
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

VEGETALE : Semences De Riz
MINISTERE DE L’AGRICULTURE REPUBLIQUE DU MALI ********* UN PEUPLE- UN BUT- UNE FOI DIRECTION NATIONALE DE L’AGRICULTURE APRAO/MALI DNA BULLETIN N°1 D’INFORMATION SUR LES SEMENCES D’ORIGINE VEGETALE : Semences de riz JANVIER 2012 1 LISTE DES ABREVIATIONS ACF : Action Contre la Faim APRAO : Amélioration de la Production de Riz en Afrique de l’Ouest CAPROSET : Centre Agro écologique de Production de Semences Tropicales CMDT : Compagnie Malienne de Développement de textile CRRA : Centre Régional de Recherche Agronomique DNA : Direction Nationale de l’Agriculture DRA : Direction Régionale de l’Agriculture ICRISAT: International Crops Research Institute for the Semi-Arid Tropics IER : Institut d’Economie Rurale IRD : International Recherche Développement MPDL : Mouvement pour le Développement Local ON : Office du Niger ONG : Organisation Non Gouvernementale OP : Organisation Paysanne PAFISEM : Projet d’Appui à la Filière Semencière du Mali PDRN : Projet de Diffusion du Riz Nérica RHK : Réseau des Horticulteurs de Kayes SSN : Service Semencier National WASA: West African Seeds Alliancy 2 INTRODUCTION Le Mali est un pays à vocation essentiellement agro pastorale. Depuis un certain temps, le Gouvernement a opté de faire du Mali une puissance agricole et faire de l’agriculture le moteur de la croissance économique. La réalisation de cette ambition passe par la combinaison de plusieurs facteurs dont la production et l’utilisation des semences certifiées. On note que la semence contribue à hauteur de 30-40% dans l’augmentation de la production agricole. En effet, les semences G4, R1 et R2 sont produites aussi bien par les structures techniques de l’Etat (Service Semencier National et l’IER) que par les sociétés et Coopératives semencières (FASO KABA, Cigogne, Comptoir 2000, etc.) ainsi que par les producteurs individuels à travers le pays. -

Situation Des Foyers De Feux De Brousse Du 14 Au 16 Fevrier 2015 Selon Le Sattelite Modis
MINISTERE DE L’ENVIRONNEMENT REPUBLIQUE DU MALI DE l’ASSAINISSEMENT ET DU DEVELOPPEMENT DURABLE UN PEUPLE/UN BUT/UNE FOI DIRECTION NATIONALE DES EAUX ET FORETS(DNEF) SYSTEME D’INFORMATION FORESTIER (SIFOR) SITUATION DES FOYERS DE FEUX DE BROUSSE DU 14 AU 16 FEVRIER 2015 SELON LE SATTELITE MODIS. LATITUDES LONGITUDES VILLAGES COMMUNES CERCLES REGIONS 12,0010000000 -10,605000000 AFFIA SAGALO KENIEBA KAYES 13,8100000000 -10,906000000 SITAFOULA MAHINA BAFOULABE KAYES 13,2420000000 -8,8870000000 DOUGOUNI B MADINA KITA KAYES 13,4790000000 -11,548000000 KENIEGOULO DIALAFARA KENIEBA KAYES 13,3550000000 -10,741000000 DIAKABA DIOKELI BAFOULABE KAYES 13,0990000000 -9,3500000000 SITANTOUNB BENDOUGOUBA KITA KAYES 13,0950000000 -9,3880000000 KOFOULABE BENDOUGOUBA KITA KAYES 12,9640000000 -9,0600000000 SANGARE BO SEBEKORO KITA KAYES 12,9440000000 -9,4230000000 GOUMBANKO BADIA KITA KAYES 13,1590000000 -11,195000000 MANAOULE KASSAMA KENIEBA KAYES 13,1420000000 -11,454000000 DJIDJAN-KE DIALAFARA KENIEBA KAYES 12,9430000000 -10,629000000 MAKADOUGOU KOUNDIAN BAFOULABE KAYES 12,7010000000 -8,9690000000 DYABALA MAKANO KITA KAYES 12,9110000000 -10,663000000 SEKOTONDIN KOUNDIAN BAFOULABE KAYES 12,7910000000 -10,082000000 KOBA KOKOFATA KITA KAYES 12,5840000000 -9,1050000000 KOTEDO SIRAKORO KITA KAYES 12,8530000000 -11,295000000 SANOUKOU KENIEBA KENIEBA KAYES 12,4400000000 -10,709000000 MADINA-TAL KOUROUKOTO KENIEBA KAYES 12,4190000000 -11,158000000 MAKOUKE FALEA KENIEBA KAYES 12,3920000000 -11,017000000 GOUBA FARABA KENIEBA KAYES 12,3100000000 -11,039000000 WILI-WILI -

Annuaire Statistique 2015 Du Secteur Développement Rural
MINISTERE DE L’AGRICULTURE REPUBLIQUE DU MALI ----------------- Un Peuple - Un But – Une Foi SECRETARIAT GENERAL ----------------- ----------------- CELLULE DE PLANIFICATION ET DE STATISTIQUE / SECTEUR DEVELOPPEMENT RURAL Annuaire Statistique 2015 du Secteur Développement Rural Juin 2016 1 LISTE DES TABLEAUX Tableau 1 : Répartition de la population par région selon le genre en 2015 ............................................................ 10 Tableau 2 : Population agricole par région selon le genre en 2015 ........................................................................ 10 Tableau 3 : Répartition de la Population agricole selon la situation de résidence par région en 2015 .............. 10 Tableau 4 : Répartition de la population agricole par tranche d'âge et par sexe en 2015 ................................. 11 Tableau 5 : Répartition de la population agricole par tranche d'âge et par Région en 2015 ...................................... 11 Tableau 6 : Population agricole par tranche d'âge et selon la situation de résidence en 2015 ............. 12 Tableau 7 : Pluviométrie décadaire enregistrée par station et par mois en 2015 ..................................................... 15 Tableau 8 : Pluviométrie décadaire enregistrée par station et par mois en 2015 (suite) ................................... 16 Tableau 9 : Pluviométrie enregistrée par mois 2015 ........................................................................................ 17 Tableau 10 : Pluviométrie enregistrée par station en 2015 et sa comparaison à -

World Bank Document
69972 Options for Preparing a Sustainable Land Management (SLM) Program in Mali Consistent with TerrAfrica for World Bank Engagement at the Country Level Introduction Public Disclosure Authorized 1. Background and rationale: 1. One of the most environmentally vulnerable areas of the world is the drylands of sub-Saharan Africa, particularly the Sahel, the Horn of Africa and Southeast Africa. Mali, as with other dryland areas in this category, suffers from droughts approximately every 30 years. These droughts triple the number of people exposed to severe water scarcity at least once in every generation, leading to major food and health crisis. In general, dryland populations lag far behind the rest of the world in human well-being and development indicators. Similarly, the average infant mortality rate for dryland developing countries exceeds that for non-dryland countries by 23% or more. The human causes of degradation1 and desertification2 include direct factors such as land use (agricultural expansion in marginal areas, deforestation, overgrazing) and indirect factors (policy failures, population pressure, land tenure). The biophysical impacts of dessertification are regional and global climate change, impairment of carbon sequestation capacity, dust storms, siltation into rivers, downstream flooding, erosion gullies and dune formation. The social impacts are devestating- increasing poverty, decreased agricultural and silvicultural production and sometimes Public Disclosure Authorized malnutrition and/or death. 2. There are clear links between land degradation and poverty. Poverty is both a cause and an effect of land degradation. Poverty drives populations to exploit their environment unsustainably because of limited resources, poorly defined property rights and limited access to credit, which prevents them from investing resources into environmental management. -

Mli0006 Ref Region De Kayes A3 15092013
MALI - Région de Kayes: Carte de référence (Septembre 2013) Limite d'Etat Limite de Région MAURITANIE Gogui Sahel Limite de Cercle Diarrah Kremis Nioro Diaye Tougoune Yerere Kirane Coura Ranga Baniere Gory Kaniaga Limite de Commune Troungoumbe Koro GUIDIME Gavinane ! Karakoro Koussane NIORO Toya Guadiaba Diafounou Guedebine Diabigue .! Chef-lieu de Région Kadiel Diongaga ! Guetema Fanga Youri Marekhaffo YELIMANE Korera Kore ! Chef-lieu de Cercle Djelebou Konsiga Bema Diafounou Fassoudebe Soumpou Gory Simby CERCLES Sero Groumera Diamanou Sandare BAFOULABE Guidimakan Tafasirga Bangassi Marintoumania Tringa Dioumara Gory Koussata DIEMA Sony Gopela Lakamane Fegui Diangounte Goumera KAYES Somankidi Marena Camara DIEMA Kouniakary Diombougou ! Khouloum KENIEBA Kemene Dianguirde KOULIKORO Faleme KAYES Diakon Gomitradougou Tambo Same .!! Sansankide Colombine Dieoura Madiga Diomgoma Lambidou KITA Hawa Segala Sacko Dembaya Fatao NIORO Logo Sidibela Tomora Sefeto YELIMANE Diallan Nord Guemoukouraba Djougoun Cette carte a été réalisée selon le découpage Diamou Sadiola Kontela administratif du Mali à partir des données de la Dindenko Sefeto Direction Nationale des Collectivités Territoriales Ouest (DNCT) BAFOULABE Kourounnikoto CERCLE COMMUNE NOM CERCLE COMMUNE NOM ! BAFOULABE KITA BAFOULABE Bafoulabé BADIA Dafela Nom de la carte: Madina BAMAFELE Diokeli BENDOUGOUBA Bendougouba DIAKON Diakon BENKADI FOUNIA Founia Moriba MLI0006 REF REGION DE KAYES A3 15092013 DIALLAN Dialan BOUDOFO Boudofo Namala DIOKELI Diokeli BOUGARIBAYA Bougarybaya Date de création: -

Academie D'enseignement De Kayes
MEN LISTE DES CANDIDATS ADMIS AU D.E.F. SESSION DE JUIN 2017 ------ PAR ORDRE ALPHABETIQUE ACADEMIE D'ENSEIGNEMENT DE KAYES. 01- CENTRE DE DIBOLI N° ANNEE STATUT PRENOMS NOM SEXE LIEU/NAIS ECOLE MENTION PLACE NAISS ELEVE 13 Ahamada Ag AMAR M 1999 Gossi CL Diboli ASSEZ-BIEN 56 Hawo BA F 2002 Nayé Peulh REG Diboli PASSABLE 219 Mahamadou B BAH M 2001 Diboli REG Diboli ASSEZ-BIEN 233 Mamadou A BAH M 2001 Diboli REG Diboli PASSABLE 288 Assétou BÄH F 2002 Diboli REG Diboli PASSABLE 292 Coumba B BAKHAYOGO F 1999 Bamako REG Diboli PASSABLE 307 Issa BALDE M 2001 Kayes REG Diboli ASSEZ-BIEN 343Sira BANE F 2001 Nayé Peulh REG Diboli PASSABLE 388 Mamadou M BARRO M 2003 Kidira REG Diboli ASSEZ-BIEN 426 Soulemane BARRY M 2000 Diboli REG Diboli PASSABLE 509 Mamadou BATHILY M 2001 Toubaboukané REG Diboli PASSABLE 999 Tidiane CAMARA M 1999 Dakassenou CL Dakassénou PASSABLE 1039Ami CISSE F 2003 Kayes REG Diboli ASSEZ-BIEN 1299 Assitan COULIBALY F 2001 Diboli REG Diboli PASSABLE 1423 Fatoumata COULIBALY F 2002 Dakassenou REG Dakassénou PASSABLE 1510 Kadiatou COULIBALY F 2001 Ségou REG Diboli PASSABLE 1542 Lassana COULIBALY M 2002 Dakassenou REG Dakassénou PASSABLE 1592 Mamadou COULIBALY M 2000 Diboli REG Diboli PASSABLE 1642 Moussa COULIBALY M 1999 Dakassenou REG Dakassénou PASSABLE 1663 Niéné M COULIBALY M 2002 Diboli REG Diboli PASSABLE 2460 Daouda DIABATE M 1999 Nayé Peulh REG Diboli PASSABLE 2561 Mamadou B DIABY M 2000 Diboli REG Diboli PASSABLE 3012 Aissé DIALLO F 2000 Diboli REG Diboli PASSABLE 3100 Cheick S DIALLO M 2002 Gakoura REG Diboli PASSABLE -

Hidden Treasure? in Search of Mali’S Gold-Mining Revenues a C I R E M a M a F X O / F F O L E T T E R B
Hidden Treasure? In search of Mali’s gold-mining revenues A C I R E M A M A F X O / F F O L E T T E R B Authors Rani Parker (Ph.D., George Washington University) is founder of Business-Community Synergies, a development economist, and a leading authority on corporate-community engagement. Parker has 20 years' experience in international development, including consultancies with organizations such as CARE, the UN Children’s Fund (UNICEF), the UN Development Program (UNDP), and multi- national companies in the energy and extractive industries. Fred Wood (Ph.D., University of London) is an internationally recognized expert on early childhood and youth development. For 12 years, Dr. Wood served as director of education for Save the Children. His consulting projects have included Save the Children, UNICEF, and the World Bank. FRONT COVER: Niama Makalu, 22, and her nephew, Amidou Dembelle, working in a field of groundnuts against the backdrop of a waste dump area for the Sadiola Hill Gold Mine in Mali. Their families were among the many people displaced when mining began in the area in the mid-1990s. Makalu, who is married to the chief of Sadiola, is now—like many others—forced to cultivate food for her family on land in close proximity to mine waste. What’s more, since the Sadiola Hill Mine now occupies much of the area’s former agricultural land, local people have no viable alternatives to farming on these few remaining sites. Above: Sign and fence on the border of the Sadiola Hill Mine, barring trespassers from land that has been conceded to the mining company. -

Admis Def 2018 Ae Nioro
LISTE DES ADMIS AU DEF 2018 - ACADEMIE D'ENSEIGNEMENT DE NIORO N° Place PRENOMS NOM Sexe Année de Naiss Lieu de Naiss. Opon DEF Statut élève Ecole CAP 117 Aliou BOLY M 2003 Fasssoudébé CLASS REG Fassoudébé 2ème C DIEMA 118 Yacouba BOLY M 2002 Fassoudébé CLASS REG Fassoudébé 2ème C DIEMA 189 Mahamadou CAMARA M 2001 Libreville CLASS REG Béma 2ème C DIEMA 262 Mariam CISSE F 2002 Fassoudébé CLASS REG Fassoudébé 2ème C DIEMA 293 Assa COULIBALY F 2002 Béma CLASS REG Béma 2ème C DIEMA 360 Konsou Fatoumata COULIBALY F 2003 Bamako CLASS REG Béma 2ème C DIEMA 388 Minata COULIBALY F 2000 M'Pèssoba CLASS CL Béma 2ème C DIEMA 496 Oumou DIA F 2004 Niantanso CLASS REG Fassoudébé 2ème C DIEMA 562 Djibril DIAKITE M 2002 Béma CLASS REG Béma 2ème C DIEMA 842 Ousmane Chérif DIARRA M 2003 Bamako CLASS REG Béma 2ème C DIEMA 905 Diougoudou DIAWARA M 2002 Libreville CLASS REG Béma 2ème C DIEMA 910 Douga DIAWARA M 2002 Béma CLASS REG Béma 2ème C DIEMA 965 Yatté DIAWARA M 2000 Grouméra CLASS REG Béma 2ème C DIEMA 1123 Moctar FOFANA M 2003 Touna CLASS REG Béma 2ème C DIEMA 1188 Foussény HAÏDARA M 2002 Béma CLASS REG Béma 2ème C DIEMA 1195 Tidiane KAH M 2003 Fassoudébé CLASS REG Fassoudébé 2ème C DIEMA 1198 Issouf KALOGA M 2000 Béma CLASS REG Béma 2ème C DIEMA 1202 Abdoulaye KAMISSOKO M 2002 Béma CLASS REG Béma 2ème C DIEMA 1205 Dengoumé KAMISSOKO M 2000 Béma CLASS REG Béma 2ème C DIEMA 1209 Komakan KAMISSOKO M 2002 Béma CLASS REG Béma 2ème C DIEMA 1211 Madi Tidiane KAMISSOKO M 2004 Bamako CLASS REG Béma 2ème C DIEMA 1241 Mohamed KANTE M 2003 Guédébiné CLASS REG -

Évaluation Finale Du Projet «Intensifier La Résilience Aux Changements
Série évaluation de projet Évaluation finale du projet «Intensifier la résilience aux changements climatiques à travers une gestion agricole et pastorale intégrée dans la zone sahélienne dans le cadre de l’approche de gestion durable des terres au Mali» Symbole du projet: GCP/MLI/038/LDF FEM ID: 4822 Annexe 4. Liste des CEAP mis en œuvre et leur situation d’accessibilité pour la visite terrain ORGANISATION DES NATIONS UNIES POUR L’ALIMENTATION ET L’AGRICULTURE Rome, 2020 Liste des CEAP du cercle de Kita Distance par rapport chef- N° Région Cercle Commune Village CEAP Observations lieu de cercle (Km) 1 Kayes Kita Kobiri Kobiri 80 Accessible 2 Kayes Kita Benkadi-founia Faraba 10 Accessible 3 Kayes Kita Bendougouba Béréla 14 Accessible 4 Kayes Kita Bendougouba Kouroula 23 Accessible 5 Kayes Kita Kassaro Balandougou-morola 80 Accessible 6 Kayes Kita Benkadi-founia Kodogoni 28 Accessible 7 Kayes Kita Bendougouba Karaya-toumoumba I 18 Accessible 8 Kayes Kita Bendougouba Karaya-toumoumba II 18 Accessible 9 Kayes Kita Bendougouba Dialaya 8 Accessible 10 Kayes Kita Sirakoro Mourgoula 62 Accessible 11 Kayes Kita Souransa-tomoto Kodala 40 Accessible 12 Kayes Kita Souransa-tomoto Kassan 20 Accessible 13 Kayes Kita Kassaro Kodialan 75 Accessible 14 Kayes Kita Djidian Kofè 23 Inaccessible 15 Kayes Kita Kita-nord Sibilikily 30 Inaccessible 16 Kayes Kita Badia Makana-bamanan 30 Inaccessible 17 Kayes Kita Badia Daféla 24 Inaccessible 18 Kayes Kita Benkadi-founia Founia-moribougou 12 Accessible 19 Kayes Kita Sirakoro Faraba II 62 Inaccessible 20 Kayes -

GE84/210 BR IFIC Nº 2747 Section Spéciale Special Section Sección
Section spéciale Index BR IFIC Nº 2747 Special Section GE84/210 Sección especial Indice International Frequency Information Circular (Terrestrial Services) ITU - Radiocommunication Bureau Circular Internacional de Información sobre Frecuencias (Servicios Terrenales) UIT - Oficina de Radiocomunicaciones Circulaire Internationale d'Information sur les Fréquences (Services de Terre) UIT - Bureau des Radiocommunications Date/Fecha : 25.06.2013 Expiry date for comments / Fecha limite para comentarios / Date limite pour les commentaires : 03.10.2013 Description of Columns / Descripción de columnas / Description des colonnes Intent Purpose of the notification Propósito de la notificación Objet de la notification 1a Assigned frequency Frecuencia asignada Fréquence assignée 4a Name of the location of Tx station Nombre del emplazamiento de estación Tx Nom de l'emplacement de la station Tx B Administration Administración Administration 4b Geographical area Zona geográfica Zone géographique 4c Geographical coordinates Coordenadas geográficas Coordonnées géographiques 6a Class of station Clase de estación Classe de station 1b Vision / sound frequency Frecuencia de portadora imagen/sonido Fréquence image / son 1ea Frequency stability Estabilidad de frecuencia Stabilité de fréquence 1e carrier frequency offset Desplazamiento de la portadora Décalage de la porteuse 7c System and colour system Sistema de transmisión / color Système et système de couleur 9d Polarization Polarización Polarisation 13c Remarks Observaciones Remarques 9 Directivity Directividad -
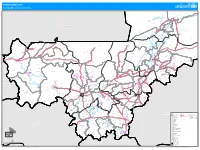
PROGRAMME ATPC Cartographie Des Interventions
PROGRAMME ATPC Cartographie des interventions Agouni !( Banikane !( TOMBOUCTOU Rharous ! Ber . !( Essakane Tin AÎcha !( Tombouctou !( Minkiri Madiakoye H! !( !( Tou!(cabangou !( Bintagoungou M'bouna Bourem-inaly !( !( Adarmalane Toya !( !( Aglal Raz-el-ma !( !( Hangabera !( Douekire GOUNDAM !( Garbakoira !( Gargando Dangha !( !( G!(ou!(ndam Sonima Doukouria Kaneye Tinguereguif Gari !( .! !( !( !( !( Kirchamba TOMBOUCTOU !( MAURITANIE Dire .! !( HaÏbongo DIRE !( Tonka Tindirma !( !( Sareyamou !( Daka Fifo Salakoira !( GOURMA-RHAROUS Kel Malha Banikane !( !( !( NIAFOUNKE Niafunke .! Soumpi Bambara Maoude !( !( Sarafere !( KoumaÏra !( Dianke I Lere !( Gogui !( !( Kormou-maraka !( N'gorkou !( N'gouma Inadiatafane Sah !( !( !( Ambiri !( Gathi-loumo !( Kirane !( Korientze Bafarara Youwarou !( Teichibe !( # YOUWAROU !( Kremis Guidi-sare !( Balle Koronga .! !( Diarra !( !( Diona !( !( Nioro Tougoune Rang Gueneibe Nampala !( Yerere Troungoumbe !( !( Ourosendegue !( !( !( !( Nioro Allahina !( Kikara .! Baniere !( Diaye Coura !( # !( Nara Dogo Diabigue !( Gavinane Guedebin!(e Korera Kore .! Bore Yelimane !( Kadiaba KadielGuetema!( !( !( !( Go!(ry Youri !( !( Fassoudebe Debere DOUENTZA !( .! !( !( !(Dallah Diongaga YELIMANE Boulal Boni !( !(Tambacara !( !( Takaba Bema # # NIORO !( # Kerena Dogofiry !( Dialloube !( !( Fanga # Dilly !( !( Kersignane !( Goumbou # KoubewelDouentza !( !( Aourou !( ## !( .! !( # K#onna Borko # # #!( !( Simbi Toguere-coumbe !( NARA !( Dogani Bere Koussane # !( !( # Dianwely-maounde # NIONO # Tongo To !( Groumera Dioura -

PROMISAM Final Technical Report, Covering the Period
Présidence de la APCAM/MSU/USAID République Projet de Mobilisation des Commissariat à la Initiatives en matière de Sécurité Alimentaire au Mali Sécurité Alimentaire (PROMISAM) PROMISAM PROJECT TO MOBILIZE FOOD SECURITY INITIATIVES IN MALI (Projet de Mobilisation des Initiatives en Matière de Sécurité Alimentaire au Mali) http://www.aec.msu.edu/agecon/fs2/mali_fd_strtgy/index.htm Final Technical Report, covering the period October 2004 – December 2007 Bamako – March 2008 This report was prepared by John Staatz, Niama Nango Dembélé, Abdramane Traoré and Valerie Kelly. PROMISAM Bamako Office ACI 2000, rue 339, porte 158 Hamdallaye Bamako, Mali Tel.: +223 222 34 19 Fax: +223 223 34 82 Name Position Email Nango Dembélé Director, COP [email protected] Abdramane Traore Project Assistant [email protected] Maïmouna Traore Admin. Asst./ Accountant [email protected] Office in the US: Department of Agricultural Economics Michigan State University 202 Agriculture Hall East Lansing, MI 48824-1039 Tel.: +1-517-355-1519 Fax: +1-517-432-1800 Contact Persons Position Email John Staatz Co-Director & Professor [email protected] Valerie Kelly Associate Professor, International Development [email protected] TABLE OF CONTENTS Executive Summary...................................................................................................................... ii 1. Background and Objectives .................................................................................................. 1 1.1 Background and Context ...............................................................................................