Topical Management of Tinea Pedis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The National Drugs List
^ ^ ^ ^ ^[ ^ The National Drugs List Of Syrian Arab Republic Sexth Edition 2006 ! " # "$ % &'() " # * +$, -. / & 0 /+12 3 4" 5 "$ . "$ 67"5,) 0 " /! !2 4? @ % 88 9 3: " # "$ ;+<=2 – G# H H2 I) – 6( – 65 : A B C "5 : , D )* . J!* HK"3 H"$ T ) 4 B K<) +$ LMA N O 3 4P<B &Q / RS ) H< C4VH /430 / 1988 V W* < C A GQ ") 4V / 1000 / C4VH /820 / 2001 V XX K<# C ,V /500 / 1992 V "!X V /946 / 2004 V Z < C V /914 / 2003 V ) < ] +$, [2 / ,) @# @ S%Q2 J"= [ &<\ @ +$ LMA 1 O \ . S X '( ^ & M_ `AB @ &' 3 4" + @ V= 4 )\ " : N " # "$ 6 ) G" 3Q + a C G /<"B d3: C K7 e , fM 4 Q b"$ " < $\ c"7: 5) G . HHH3Q J # Hg ' V"h 6< G* H5 !" # $%" & $' ,* ( )* + 2 ا اوا ادو +% 5 j 2 i1 6 B J' 6<X " 6"[ i2 "$ "< * i3 10 6 i4 11 6! ^ i5 13 6<X "!# * i6 15 7 G!, 6 - k 24"$d dl ?K V *4V h 63[46 ' i8 19 Adl 20 "( 2 i9 20 G Q) 6 i10 20 a 6 m[, 6 i11 21 ?K V $n i12 21 "% * i13 23 b+ 6 i14 23 oe C * i15 24 !, 2 6\ i16 25 C V pq * i17 26 ( S 6) 1, ++ &"r i19 3 +% 27 G 6 ""% i19 28 ^ Ks 2 i20 31 % Ks 2 i21 32 s * i22 35 " " * i23 37 "$ * i24 38 6" i25 39 V t h Gu* v!* 2 i26 39 ( 2 i27 40 B w< Ks 2 i28 40 d C &"r i29 42 "' 6 i30 42 " * i31 42 ":< * i32 5 ./ 0" -33 4 : ANAESTHETICS $ 1 2 -1 :GENERAL ANAESTHETICS AND OXYGEN 4 $1 2 2- ATRACURIUM BESYLATE DROPERIDOL ETHER FENTANYL HALOTHANE ISOFLURANE KETAMINE HCL NITROUS OXIDE OXYGEN PROPOFOL REMIFENTANIL SEVOFLURANE SUFENTANIL THIOPENTAL :LOCAL ANAESTHETICS !67$1 2 -5 AMYLEINE HCL=AMYLOCAINE ARTICAINE BENZOCAINE BUPIVACAINE CINCHOCAINE LIDOCAINE MEPIVACAINE OXETHAZAINE PRAMOXINE PRILOCAINE PREOPERATIVE MEDICATION & SEDATION FOR 9*: ;< " 2 -8 : : SHORT -TERM PROCEDURES ATROPINE DIAZEPAM INJ. -

Terbinafine-Induced Taste Impairment - Report of Two Cases Rajesh Sinha, Pallavi Sharma, Pramod Kumar, Vaibhav Kuchhal*
Journal of Pakistan Association of Dermatologists 2012; 22 (4):363-365. Case Report Terbinafine-induced taste impairment - report of two cases Rajesh Sinha, Pallavi Sharma, Pramod Kumar, Vaibhav Kuchhal* Department of Skin &V.D., Government Medical College, Haldwani 263139, Uttarakhand, India *Department of E.N.T., Government Medical College, Haldwani 263139, Uttarakhand, India Abstract Terbinafine is an oral antimycotic agent that belongs to the allylamine class. It was introduced in 1991 and is being widely used, both topically and systemically, to treat fungal infections. Nowadays oral terbinafine has become a commonly prescribed drug to treat finger- and toenail fungal infections because of relatively short duration of treatment compared to other oral antifungals like griseofulvin and fluconazole. The common side effects of this drug include nausea, abdominal pain, elevated transaminases and allergic reactions. Loss of taste sensation is a rare side effect occurring in patient taking oral form of this drug. PubMed search showed that very few cases of terbinafine- induced taste loss have been reported worldwide. We report a case series of two patients who complained of taste loss after taking terbinafine. Key words Terbinafine, ageusia. Introduction terbinafine that occurs in 0.6% to 2.8% of patients taking the drug. A low BMI, ageing Terbinafine is a widely prescribed oral and history of taste loss are considered risk antifungal agent. Its mode of action is by factors for developing taste loss due to inhibiting squalene epoxidase which converts terbinafine. squalene to lanosterol. With a decrease in lanosterol production, ergosterol production is Case report 1 also diminished, affecting fungal cell membrane synthesis and function. -

Long-Term Effectiveness of Treatment with Terbinafine Vs Itraconazole in Onychomycosis a 5-Year Blinded Prospective Follow-Up Study
STUDY Long-term Effectiveness of Treatment With Terbinafine vs Itraconazole in Onychomycosis A 5-Year Blinded Prospective Follow-up Study Ba´rður Sigurgeirsson, MD, PhD; Jo´n H. O´ lafsson, MD, PhD; Jo´n þ. Steinsson, MD Carle Paul, MD; Stephan Billstein, MD; E. Glyn V. Evans, PhD Objective: To examine long-term cure and relapse rates microscopy and culture at the end of follow-up and no re- after treatment with continuous terbinafine and inter- quirement of second intervention treatment. Secondary ef- mittent itraconazole in onychomycosis. ficacy criteria included clinical cure without second inter- vention treatment and mycological and clinical relapse rates. Design: Long-term prospective follow-up study. Results: Median duration of follow-up was 54 months. Setting: Three centers in Iceland. At the end of the study, mycological cure without second intervention treatment was found in 34 (46%) of the 74 Subjects: The study population comprised 151 pa- terbinafine-treated subjects and 10 (13%) of the 77 itra- tients aged 18 to 75 years with a clinical and mycologi- conazole-treated subjects (PϽ.001). Mycological and clini- cal diagnosis of dermatophyte toenail onychomycosis. cal relapse rates were significantly higher in itraconazole- vs terbinafine-treated patients (53% vs 23% and 48% vs Interventions: In a double-blind, double-dummy study, 21%, respectively). Of the 72 patients who received sub- patients were randomized to receive either terbinafine (250 sequent terbinafine treatment, 63 (88%) achieved myco- mg/d) for 12 or 16 weeks or itraconazole (400 mg/d) for logical cure and 55 (76%) achieved clinical cure. 1 week in every 4 for 12 or 16 weeks (first intervention). -

Diagnosis and Treatment of Tinea Versicolor Ronald Savin, MD New Haven, Connecticut
■ CLINICAL REVIEW Diagnosis and Treatment of Tinea Versicolor Ronald Savin, MD New Haven, Connecticut Tinea versicolor (pityriasis versicolor) is a common imidazole, has been used for years both orally and top superficial fungal infection of the stratum corneum. ically with great success, although it has not been Caused by the fungus Malassezia furfur, this chronical approved by the Food and Drug Administration for the ly recurring disease is most prevalent in the tropics but indication of tinea versicolor. Newer derivatives, such is also common in temperate climates. Treatments are as fluconazole and itraconazole, have recently been available and cure rates are high, although recurrences introduced. Side effects associated with these triazoles are common. Traditional topical agents such as seleni tend to be minor and low in incidence. Except for keto um sulfide are effective, but recurrence following treat conazole, oral antifungals carry a low risk of hepato- ment with these agents is likely and often rapid. toxicity. Currently, therapeutic interest is focused on synthetic Key Words: Tinea versicolor; pityriasis versicolor; anti “-azole” antifungal drugs, which interfere with the sterol fungal agents. metabolism of the infectious agent. Ketoconazole, an (J Fam Pract 1996; 43:127-132) ormal skin flora includes two morpho than formerly thought. In one study, children under logically discrete lipophilic yeasts: a age 14 represented nearly 5% of confirmed cases spherical form, Pityrosporum orbicu- of the disease.3 In many of these cases, the face lare, and an ovoid form, Pityrosporum was involved, a rare manifestation of the disease in ovale. Whether these are separate enti adults.1 The condition is most prevalent in tropical tiesN or different morphologic forms in the cell and semitropical areas, where up to 40% of some cycle of the same organism remains unclear.: In the populations are affected. -

Step Therapy Criteria Drug Class Topical Antifungal Agents (Brand Products Only)
STEP THERAPY CRITERIA DRUG CLASS TOPICAL ANTIFUNGAL AGENTS (BRAND PRODUCTS ONLY) BRAND NAME (generic) ECOZA (econazole) ERTACZO (sertaconazole) EXELDERM (sulconazole nitrate) LOPROX (ciclopirox shampoo) LOTRISONE (clotrimazole/betamethasone) LUZU (luliconazole) MENTAX (butenafine) NAFTIN (naftifine) OXISTAT (oxiconazole) VUSION (miconazole/zinc oxide/white petrolatum) XOLEGEL (ketoconazole) Antifungal Topical Step Therapy Policy 06-2017 CVS Caremark is an independent company that provides pharmacy benefit management services to CareFirst BlueCross BlueShield and CareFirst BlueChoice, Inc. members. CareFirst BlueCross BlueShield is the shared business name of CareFirst of Maryland, Inc. and Group Hospitalization and Medical Services, Inc. CareFirst of Maryland, Inc., Group Hospitalization and Medical Services, Inc., CareFirst BlueChoice, Inc., The Dental Network and First Care, Inc. are independent licensees of the Blue Cross and Blue Shield Association. In the District of Columbia and Maryland, CareFirst MedPlus is the business name of First Care, Inc. In Virginia, CareFirst MedPlus is the business name of First Care, Inc. of Maryland (used in VA by: First Care, Inc.). ® Registered trademark of the Blue Cross and Blue Shield Association Page 1 of 4 Status: CVS Caremark Criteria Type: Initial Step Therapy; Post Step Therapy Prior Authorization POLICY FDA-APPROVED INDICATIONS Ecoza Ecoza topical 1% foam is indicated for the treatment of interdigital tinea pedis caused by Trichophyton rubrum, Trichophyton mentagrophytes, and Epidermophyton -

Therapeutic Class Overview Antifungals, Topical
Therapeutic Class Overview Antifungals, Topical INTRODUCTION The topical antifungals are available in multiple dosage forms and are indicated for a number of fungal infections and related conditions. In general, these agents are Food and Drug Administration (FDA)-approved for the treatment of cutaneous candidiasis, onychomycosis, seborrheic dermatitis, tinea corporis, tinea cruris, tinea pedis, and tinea versicolor (Clinical Pharmacology 2018). The antifungals may be further classified into the following categories based upon their chemical structures: allylamines (naftifine, terbinafine [only available over the counter (OTC)]), azoles (clotrimazole, econazole, efinaconazole, ketoconazole, luliconazole, miconazole, oxiconazole, sertaconazole, sulconazole), benzylamines (butenafine), hydroxypyridones (ciclopirox), oxaborole (tavaborole), polyenes (nystatin), thiocarbamates (tolnaftate [no FDA-approved formulations]), and miscellaneous (undecylenic acid [no FDA-approved formulations]) (Micromedex 2018). The topical antifungals are available as single entity and/or combination products. Two combination products, nystatin/triamcinolone and Lotrisone (clotrimazole/betamethasone), contain an antifungal and a corticosteroid preparation. The corticosteroid helps to decrease inflammation and indirectly hasten healing time. The other combination product, Vusion (miconazole/zinc oxide/white petrolatum), contains an antifungal and zinc oxide. Zinc oxide acts as a skin protectant and mild astringent with weak antiseptic properties and helps to -

Estonian Statistics on Medicines 2016 1/41
Estonian Statistics on Medicines 2016 ATC code ATC group / Active substance (rout of admin.) Quantity sold Unit DDD Unit DDD/1000/ day A ALIMENTARY TRACT AND METABOLISM 167,8985 A01 STOMATOLOGICAL PREPARATIONS 0,0738 A01A STOMATOLOGICAL PREPARATIONS 0,0738 A01AB Antiinfectives and antiseptics for local oral treatment 0,0738 A01AB09 Miconazole (O) 7088 g 0,2 g 0,0738 A01AB12 Hexetidine (O) 1951200 ml A01AB81 Neomycin+ Benzocaine (dental) 30200 pieces A01AB82 Demeclocycline+ Triamcinolone (dental) 680 g A01AC Corticosteroids for local oral treatment A01AC81 Dexamethasone+ Thymol (dental) 3094 ml A01AD Other agents for local oral treatment A01AD80 Lidocaine+ Cetylpyridinium chloride (gingival) 227150 g A01AD81 Lidocaine+ Cetrimide (O) 30900 g A01AD82 Choline salicylate (O) 864720 pieces A01AD83 Lidocaine+ Chamomille extract (O) 370080 g A01AD90 Lidocaine+ Paraformaldehyde (dental) 405 g A02 DRUGS FOR ACID RELATED DISORDERS 47,1312 A02A ANTACIDS 1,0133 Combinations and complexes of aluminium, calcium and A02AD 1,0133 magnesium compounds A02AD81 Aluminium hydroxide+ Magnesium hydroxide (O) 811120 pieces 10 pieces 0,1689 A02AD81 Aluminium hydroxide+ Magnesium hydroxide (O) 3101974 ml 50 ml 0,1292 A02AD83 Calcium carbonate+ Magnesium carbonate (O) 3434232 pieces 10 pieces 0,7152 DRUGS FOR PEPTIC ULCER AND GASTRO- A02B 46,1179 OESOPHAGEAL REFLUX DISEASE (GORD) A02BA H2-receptor antagonists 2,3855 A02BA02 Ranitidine (O) 340327,5 g 0,3 g 2,3624 A02BA02 Ranitidine (P) 3318,25 g 0,3 g 0,0230 A02BC Proton pump inhibitors 43,7324 A02BC01 Omeprazole -

Nails: Tales, Fails and What Prevails in Treating Onychomycosis
J. Hibler, D.O. OhioHealth - O’Bleness Memorial Hospital, Athens, Ohio AOCD Annual Conference Orlando, Florida 10.18.15 A) Onychodystrophy B) Onychogryphosis C)“Question Onychomycosis dogma” – Michael Conroy, MD D) All the above E) None of the above Nail development begins at 8-10 weeks EGA Complete by 5th month Keratinization ~11 weeks No granular layer Nail plate growth: Fingernails 3 mm/month, toenails 1 mm/month Faster in summer or winter? Summer! Index finger or 5th digit nail grows faster? Index finger! Faster growth to middle or lateral edge of each nail? Lateral! Elkonyxis Mee’s lines Aka leukonychia striata Arsenic poisoning Trauma Medications Illness Psoriasis flare Muerhrcke’s bands Hypoalbuminemia Chemotherapy Half & half nails Aka Lindsay’s nails Chronic renal disease Terry’s nails Liver failure, Cirrhosis Malnutrition Diabetes Cardiovascular disease True or False: Onychomycosis = Tinea Unguium? FALSE. Onychomycosis: A fungal disease of the nails (all causes) Dermatophytes, yeasts, molds Tinea unguium: A fungal disease of nail caused by dermatophyte fungi Onychodystrophy ≠ onychomycosis Accounts for up to 50% of all nail disorders Prevalence; 14-28% of > 60 year-olds Variety of subtypes; know them! Sequelae What is the most common cause of onychomycosis? A) Epidermophyton floccosum B) Microsporum spp C) Trichophyton mentagrophytes D) Trichophyton rubrum -Account for ~90% of infections Dermatophytes Trichophyton rubrum Trichophyton mentagrophytes Trichophyton tonsurans, Microsporum canis, Epidermophyton floccosum Nondermatophyte molds Acremonium spp, Fusarium spp Scopulariopsis spp, Sytalidium spp, Aspergillus spp Yeast Candida parapsilosis Candida albicans Candida spp Distal/lateral subungal Proximal subungual onychomycosis onychomycosis (DLSO) (PSO) Most common; T. rubrum Often in immunosuppressed patients T. -

Treatment of Interdigital Tinea Pedis with 25% and 50% Tea Tree Oil Solution: a Randomized, Placebo-Controlled, Blinded Study
Australasian Journal of Dermatology (2002) 43, 175–178 RESEARCH REPORT Treatment of interdigital tinea pedis with 25% and 50% tea tree oil solution: A randomized, placebo-controlled, blinded study Andrew C Satchell,1 Anne Saurajen,1 Craig Bell2 and Ross StC Barnetson1 1Department of Dermatology, Royal Prince Alfred Hospital, Camperdown and 2Australian Tea Tree Oil Research Institute, Southern Cross University, Lismore, New South Wales, Australia and Epidermophyton floccosum, and appears to be related 2 SUMMARY to occlusive footwear. Tinea pedis occurs as one of four clinical variants: intertriginous, papulosquamous, vesicular Tea tree oil has been shown to have activity against and acute ulcerative.3 Chronic, intertriginous tinea pedis is dermatophytes in vitro. We have conducted a random- characterized by a scaling and fissuring of the lateral toe ized, controlled, double-blinded study to determine the webs caused by dermatophyte invasion of the stratum efficacy and safety of 25% and 50% tea tree oil in corneum; macerated, erosive infections may follow as a result the treatment of interdigital tinea pedis. One hundred of secondary overgrowth of commensal bacteria, including and fifty-eight patients with tinea pedis clinically and Micrococcaceae (usually staphylococci), aerobic coryneforms microscopy suggestive of a dermatophyte infection and Gram-negative organisms.4 Microscopy and culture of were randomized to receive either placebo, 25% or 50% skin scrapings are used to identify the relevant organism. tea tree oil solution. Patients applied the solution twice The treatment of intertiginous tinea pedis includes daily to affected areas for 4 weeks and were reviewed measures aimed at reducing hyperhidrosis, such as talcum after 2 and 4 weeks of treatment. -

Terbinafine (TER-Bin-A-Feen)/ Urea (Ure-EE-A)/Thymol
Amphotericin B (am-foe-TER-i-sin B)/ Terbinafine (TER-bin-a-feen)/ Urea (ure-EE-a)/Thymol Treats a fungus infection on your skin, including tinea pedis (“athlete’s foot”), Treats certain skin and nail conditions. How to Use This Medicine: Cream • Your doctor will tell you how much of this medicine to apply and how often. Do not use more medicine or apply it more often than your doctor tells you to. • Follow the instructions on the medicine label if you are using this medicine without a prescription. • Use this medicine only on your skin or nails. Rinse it off right away if it gets on a cut or scrape. Do not get the medicine in your eyes, nose, or mouth. • Before using this medicine, use soap and water to wash the skin where you will use the medicine. Dry your skin with a clean towel. • Put a thin layer of the cream over the infected area and rub it in gently. • Do not cover the treated area with a bandage unless directed by your doctor. • When applying topical preparations to nails, let drug dry uncovered, unless otherwise directed by your doctor. • Take all of the medicine in your prescription to clear up your infection, even if you feel better after the first few doses. • Wash your hands with soap and water before and after you use this medicine. • Do not apply cosmetics, or other skin products to treated skin areas when using topical preparations. Avoid drug contact with eyes, lips, or mucous membranes. Possible Side Effects While Using This Medicine: Call your doctor right away if you notice any of these side effects: • Itching, rash, swelling, blisters, or redness that were not there before you used this medicine • Drug may cause sun sensitivity. -

Fluconazole, Griseofulvin, Itraconazole, Terbinafine) in Current Epidemic of Altered Dermatophytosis in India: a Randomized Pragmatic Trial
medRxiv preprint doi: https://doi.org/10.1101/19003541; this version posted August 2, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. It is made available under a CC-BY-NC-ND 4.0 International license . 1 Title Effectiveness of four oral antifungal drugs (fluconazole, griseofulvin, itraconazole, terbinafine) in current epidemic of altered dermatophytosis in India: A randomized pragmatic trial Authors 1. Sanjay Singh, MD, DNB, PhD 2. Usha Chandra, MD 3. Priyanka Verma, MD 4. Vinayak N Anchan, MBBS 5. Ragini Tilaka, MD Departments of Dermatology and Venereology, and aMicrobiology, Institute of Medical Sciences, Banaras Hindu University, Varanasi, India Sources of support: Banaras Hindu University Address for correspondence: Dr Sanjay Singh, Professor and Head, Department of Dermatology and Venereology, Institute of Medical Sciences, Banaras Hindu University, Varanasi, India. Email: [email protected] One Sentence Summary: Effectiveness of all four antifungals has declined, with itraconazole being the most effective currently in dermatophytosis in India. NOTE: This preprint reports new research that has not been certified by peer review and should not be used to guide clinical practice. medRxiv preprint doi: https://doi.org/10.1101/19003541; this version posted August 2, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. It is made available under a CC-BY-NC-ND 4.0 International license . 2 Abstract Background: Dermatophyte infections have undergone unprecedented changes in India in recent past. -
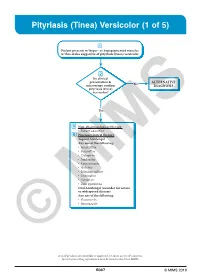
Pityriasis (Tinea) Versicolor (1 of 5)
Pityriasis (Tinea) Versicolor (1 of 5) 1 Patient presents w/ hyper- or hypopigmented macules w/ fine scales suggestive of pityriasis (tinea) versicolor 2 Do clinical presentation & No ALTERNATIVE microscopy confirm DIAGNOSIS pityriasis (tinea) versicolor? Yes A Non-pharmacological therapy • Patient education B Pharmacological therapy Topical Antifungal Any one of the following: • Amorolfi ne • Butenafi ne • Ciclopirox • Imidazoles • Ketoconazole • Naftifi ne • Selenium sulfi de • Terbinafi ne • Tolnaftate • Zinc pyrithione Oral Antifungal (consider for severe or widespread disease) Any one of the following: • Fluconazole • Itraconazole © MIMS Not all products are available or approved for above use in all countries. Specifi c prescribing information may be found in the latest MIMS. B307 © MIMS 2019 Pityriasis (Tinea) Versicolor (2 of 5) 1 EVALUATION Pityriasis (tinea) versicolor is a common, benign, superfi cial fungal infection localized to the stratum corneum • Caused by lipophilic yeasts, Malassezia species, part of the normal fl ora of the human skin Clinical Presentation • May present as chronic or recurrent infection & may occur in healthy individuals - More common in summer than winter months • Predominates in young adults when the sebaceous glands are most active • Presents w/ multiple well-demarcated macules or patches & fi nely scaled plaques w/ hypopigmentation or hyperpigmentation, hence the term “versicolor” • Tends to be asymptomatic & is mainly a cosmetic concern but pruritus may or may not be present • Usually found on the upper trunk,