Clinical Review
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The National Drugs List
^ ^ ^ ^ ^[ ^ The National Drugs List Of Syrian Arab Republic Sexth Edition 2006 ! " # "$ % &'() " # * +$, -. / & 0 /+12 3 4" 5 "$ . "$ 67"5,) 0 " /! !2 4? @ % 88 9 3: " # "$ ;+<=2 – G# H H2 I) – 6( – 65 : A B C "5 : , D )* . J!* HK"3 H"$ T ) 4 B K<) +$ LMA N O 3 4P<B &Q / RS ) H< C4VH /430 / 1988 V W* < C A GQ ") 4V / 1000 / C4VH /820 / 2001 V XX K<# C ,V /500 / 1992 V "!X V /946 / 2004 V Z < C V /914 / 2003 V ) < ] +$, [2 / ,) @# @ S%Q2 J"= [ &<\ @ +$ LMA 1 O \ . S X '( ^ & M_ `AB @ &' 3 4" + @ V= 4 )\ " : N " # "$ 6 ) G" 3Q + a C G /<"B d3: C K7 e , fM 4 Q b"$ " < $\ c"7: 5) G . HHH3Q J # Hg ' V"h 6< G* H5 !" # $%" & $' ,* ( )* + 2 ا اوا ادو +% 5 j 2 i1 6 B J' 6<X " 6"[ i2 "$ "< * i3 10 6 i4 11 6! ^ i5 13 6<X "!# * i6 15 7 G!, 6 - k 24"$d dl ?K V *4V h 63[46 ' i8 19 Adl 20 "( 2 i9 20 G Q) 6 i10 20 a 6 m[, 6 i11 21 ?K V $n i12 21 "% * i13 23 b+ 6 i14 23 oe C * i15 24 !, 2 6\ i16 25 C V pq * i17 26 ( S 6) 1, ++ &"r i19 3 +% 27 G 6 ""% i19 28 ^ Ks 2 i20 31 % Ks 2 i21 32 s * i22 35 " " * i23 37 "$ * i24 38 6" i25 39 V t h Gu* v!* 2 i26 39 ( 2 i27 40 B w< Ks 2 i28 40 d C &"r i29 42 "' 6 i30 42 " * i31 42 ":< * i32 5 ./ 0" -33 4 : ANAESTHETICS $ 1 2 -1 :GENERAL ANAESTHETICS AND OXYGEN 4 $1 2 2- ATRACURIUM BESYLATE DROPERIDOL ETHER FENTANYL HALOTHANE ISOFLURANE KETAMINE HCL NITROUS OXIDE OXYGEN PROPOFOL REMIFENTANIL SEVOFLURANE SUFENTANIL THIOPENTAL :LOCAL ANAESTHETICS !67$1 2 -5 AMYLEINE HCL=AMYLOCAINE ARTICAINE BENZOCAINE BUPIVACAINE CINCHOCAINE LIDOCAINE MEPIVACAINE OXETHAZAINE PRAMOXINE PRILOCAINE PREOPERATIVE MEDICATION & SEDATION FOR 9*: ;< " 2 -8 : : SHORT -TERM PROCEDURES ATROPINE DIAZEPAM INJ. -

Step Therapy Criteria Drug Class Topical Antifungal Agents (Brand Products Only)
STEP THERAPY CRITERIA DRUG CLASS TOPICAL ANTIFUNGAL AGENTS (BRAND PRODUCTS ONLY) BRAND NAME (generic) ECOZA (econazole) ERTACZO (sertaconazole) EXELDERM (sulconazole nitrate) LOPROX (ciclopirox shampoo) LOTRISONE (clotrimazole/betamethasone) LUZU (luliconazole) MENTAX (butenafine) NAFTIN (naftifine) OXISTAT (oxiconazole) VUSION (miconazole/zinc oxide/white petrolatum) XOLEGEL (ketoconazole) Antifungal Topical Step Therapy Policy 06-2017 CVS Caremark is an independent company that provides pharmacy benefit management services to CareFirst BlueCross BlueShield and CareFirst BlueChoice, Inc. members. CareFirst BlueCross BlueShield is the shared business name of CareFirst of Maryland, Inc. and Group Hospitalization and Medical Services, Inc. CareFirst of Maryland, Inc., Group Hospitalization and Medical Services, Inc., CareFirst BlueChoice, Inc., The Dental Network and First Care, Inc. are independent licensees of the Blue Cross and Blue Shield Association. In the District of Columbia and Maryland, CareFirst MedPlus is the business name of First Care, Inc. In Virginia, CareFirst MedPlus is the business name of First Care, Inc. of Maryland (used in VA by: First Care, Inc.). ® Registered trademark of the Blue Cross and Blue Shield Association Page 1 of 4 Status: CVS Caremark Criteria Type: Initial Step Therapy; Post Step Therapy Prior Authorization POLICY FDA-APPROVED INDICATIONS Ecoza Ecoza topical 1% foam is indicated for the treatment of interdigital tinea pedis caused by Trichophyton rubrum, Trichophyton mentagrophytes, and Epidermophyton -

Therapeutic Class Overview Antifungals, Topical
Therapeutic Class Overview Antifungals, Topical INTRODUCTION The topical antifungals are available in multiple dosage forms and are indicated for a number of fungal infections and related conditions. In general, these agents are Food and Drug Administration (FDA)-approved for the treatment of cutaneous candidiasis, onychomycosis, seborrheic dermatitis, tinea corporis, tinea cruris, tinea pedis, and tinea versicolor (Clinical Pharmacology 2018). The antifungals may be further classified into the following categories based upon their chemical structures: allylamines (naftifine, terbinafine [only available over the counter (OTC)]), azoles (clotrimazole, econazole, efinaconazole, ketoconazole, luliconazole, miconazole, oxiconazole, sertaconazole, sulconazole), benzylamines (butenafine), hydroxypyridones (ciclopirox), oxaborole (tavaborole), polyenes (nystatin), thiocarbamates (tolnaftate [no FDA-approved formulations]), and miscellaneous (undecylenic acid [no FDA-approved formulations]) (Micromedex 2018). The topical antifungals are available as single entity and/or combination products. Two combination products, nystatin/triamcinolone and Lotrisone (clotrimazole/betamethasone), contain an antifungal and a corticosteroid preparation. The corticosteroid helps to decrease inflammation and indirectly hasten healing time. The other combination product, Vusion (miconazole/zinc oxide/white petrolatum), contains an antifungal and zinc oxide. Zinc oxide acts as a skin protectant and mild astringent with weak antiseptic properties and helps to -

Estonian Statistics on Medicines 2016 1/41
Estonian Statistics on Medicines 2016 ATC code ATC group / Active substance (rout of admin.) Quantity sold Unit DDD Unit DDD/1000/ day A ALIMENTARY TRACT AND METABOLISM 167,8985 A01 STOMATOLOGICAL PREPARATIONS 0,0738 A01A STOMATOLOGICAL PREPARATIONS 0,0738 A01AB Antiinfectives and antiseptics for local oral treatment 0,0738 A01AB09 Miconazole (O) 7088 g 0,2 g 0,0738 A01AB12 Hexetidine (O) 1951200 ml A01AB81 Neomycin+ Benzocaine (dental) 30200 pieces A01AB82 Demeclocycline+ Triamcinolone (dental) 680 g A01AC Corticosteroids for local oral treatment A01AC81 Dexamethasone+ Thymol (dental) 3094 ml A01AD Other agents for local oral treatment A01AD80 Lidocaine+ Cetylpyridinium chloride (gingival) 227150 g A01AD81 Lidocaine+ Cetrimide (O) 30900 g A01AD82 Choline salicylate (O) 864720 pieces A01AD83 Lidocaine+ Chamomille extract (O) 370080 g A01AD90 Lidocaine+ Paraformaldehyde (dental) 405 g A02 DRUGS FOR ACID RELATED DISORDERS 47,1312 A02A ANTACIDS 1,0133 Combinations and complexes of aluminium, calcium and A02AD 1,0133 magnesium compounds A02AD81 Aluminium hydroxide+ Magnesium hydroxide (O) 811120 pieces 10 pieces 0,1689 A02AD81 Aluminium hydroxide+ Magnesium hydroxide (O) 3101974 ml 50 ml 0,1292 A02AD83 Calcium carbonate+ Magnesium carbonate (O) 3434232 pieces 10 pieces 0,7152 DRUGS FOR PEPTIC ULCER AND GASTRO- A02B 46,1179 OESOPHAGEAL REFLUX DISEASE (GORD) A02BA H2-receptor antagonists 2,3855 A02BA02 Ranitidine (O) 340327,5 g 0,3 g 2,3624 A02BA02 Ranitidine (P) 3318,25 g 0,3 g 0,0230 A02BC Proton pump inhibitors 43,7324 A02BC01 Omeprazole -
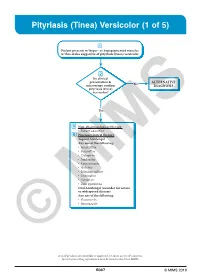
Pityriasis (Tinea) Versicolor (1 of 5)
Pityriasis (Tinea) Versicolor (1 of 5) 1 Patient presents w/ hyper- or hypopigmented macules w/ fine scales suggestive of pityriasis (tinea) versicolor 2 Do clinical presentation & No ALTERNATIVE microscopy confirm DIAGNOSIS pityriasis (tinea) versicolor? Yes A Non-pharmacological therapy • Patient education B Pharmacological therapy Topical Antifungal Any one of the following: • Amorolfi ne • Butenafi ne • Ciclopirox • Imidazoles • Ketoconazole • Naftifi ne • Selenium sulfi de • Terbinafi ne • Tolnaftate • Zinc pyrithione Oral Antifungal (consider for severe or widespread disease) Any one of the following: • Fluconazole • Itraconazole © MIMS Not all products are available or approved for above use in all countries. Specifi c prescribing information may be found in the latest MIMS. B307 © MIMS 2019 Pityriasis (Tinea) Versicolor (2 of 5) 1 EVALUATION Pityriasis (tinea) versicolor is a common, benign, superfi cial fungal infection localized to the stratum corneum • Caused by lipophilic yeasts, Malassezia species, part of the normal fl ora of the human skin Clinical Presentation • May present as chronic or recurrent infection & may occur in healthy individuals - More common in summer than winter months • Predominates in young adults when the sebaceous glands are most active • Presents w/ multiple well-demarcated macules or patches & fi nely scaled plaques w/ hypopigmentation or hyperpigmentation, hence the term “versicolor” • Tends to be asymptomatic & is mainly a cosmetic concern but pruritus may or may not be present • Usually found on the upper trunk, -

Estonian Statistics on Medicines 2013 1/44
Estonian Statistics on Medicines 2013 DDD/1000/ ATC code ATC group / INN (rout of admin.) Quantity sold Unit DDD Unit day A ALIMENTARY TRACT AND METABOLISM 146,8152 A01 STOMATOLOGICAL PREPARATIONS 0,0760 A01A STOMATOLOGICAL PREPARATIONS 0,0760 A01AB Antiinfectives and antiseptics for local oral treatment 0,0760 A01AB09 Miconazole(O) 7139,2 g 0,2 g 0,0760 A01AB12 Hexetidine(O) 1541120 ml A01AB81 Neomycin+Benzocaine(C) 23900 pieces A01AC Corticosteroids for local oral treatment A01AC81 Dexamethasone+Thymol(dental) 2639 ml A01AD Other agents for local oral treatment A01AD80 Lidocaine+Cetylpyridinium chloride(gingival) 179340 g A01AD81 Lidocaine+Cetrimide(O) 23565 g A01AD82 Choline salicylate(O) 824240 pieces A01AD83 Lidocaine+Chamomille extract(O) 317140 g A01AD86 Lidocaine+Eugenol(gingival) 1128 g A02 DRUGS FOR ACID RELATED DISORDERS 35,6598 A02A ANTACIDS 0,9596 Combinations and complexes of aluminium, calcium and A02AD 0,9596 magnesium compounds A02AD81 Aluminium hydroxide+Magnesium hydroxide(O) 591680 pieces 10 pieces 0,1261 A02AD81 Aluminium hydroxide+Magnesium hydroxide(O) 1998558 ml 50 ml 0,0852 A02AD82 Aluminium aminoacetate+Magnesium oxide(O) 463540 pieces 10 pieces 0,0988 A02AD83 Calcium carbonate+Magnesium carbonate(O) 3049560 pieces 10 pieces 0,6497 A02AF Antacids with antiflatulents Aluminium hydroxide+Magnesium A02AF80 1000790 ml hydroxide+Simeticone(O) DRUGS FOR PEPTIC ULCER AND GASTRO- A02B 34,7001 OESOPHAGEAL REFLUX DISEASE (GORD) A02BA H2-receptor antagonists 3,5364 A02BA02 Ranitidine(O) 494352,3 g 0,3 g 3,5106 A02BA02 Ranitidine(P) -

Pepper and Garlic Extracts As an Alternative Treatment To
ISSN Online: 2378-1726 Symbiosis www.symbiosisonlinepublishing.com Opinion Article Clinical Research in Dermatology: Open Access Open Access Pepper and Garlic Extracts as an Alternative Treatment to Onychomycosis: AMyth or Truth? Hsuan-Hsiang Chen* Department of Dermatology, National Taiwan University Hospital and National Taiwan University College of Medicine, Taipei, TaiwanMiami, Miller School of Medicine, Miami, FL, USA. Received: May 25, 2021; Accepted: May 29, 2021; Published: June 01, 2021 *Corresponding author: Hsuan-Hsiang Chen, MD,Department of Dermatology, National Taiwan University Hospital, 7, Chung-Shan South Road,Taipei, Taiwan, Tel. No: +886-2-2356-2141; Fax. No: +886-2-2393-4177, E-mail: [email protected] Onychomycosis is a chronic disease ofa nail infection by the germinationcapacity. In vivo, the antioxidant activityof A. sativum fungi. Based on the mode of nail invasion and morphologic patterns, it can be divided into 5subtypes: distal and lateral subungualonychomycosis, proximal subungualonychomycosis, associatedextract may with be effective onychomycosis. as a local or systemic anti-inflammatory therapy and target the oxidative stress from the inflammation The A.sativum extract is effective against Meyerozymaguilliermondii and endonyxsubungualonychomycosis.Common pathogens and Rhodotorulamucilaginosa cultured from the toenails of a aresuperficial dermatophytes, onychomycosis, non-dermatophyte total dystrophic molds, onychomycosis,and yeasts. In patient with onychomycosis[5]. Parvu et al. demonstrated the recent years, the development of new antifungal agents, such as allylamines and azoles, has provided good treatment options for Venugopal et al. also investigated the anti-dermatophyte activity onychomycosistherapy. ofantifungal the aqueous effect extract of 4% ofgarlic A. sativum extract against was comparable 88 clinical to isolates naftifine. of There is a growing interest in the use of plant-derived compounds dermatophytes[6]. -

Appendix B - Product Name Sorted by Applicant
JUNE 2021 - APPROVED DRUG PRODUCT LIST B - 1 APPENDIX B - PRODUCT NAME SORTED BY APPLICANT ** 3 ** 3D IMAGING DRUG * 3D IMAGING DRUG DESIGN AND DEVELOPMENT LLC AMMONIA N 13, AMMONIA N-13 FLUDEOXYGLUCOSE F18, FLUDEOXYGLUCOSE F-18 SODIUM FLUORIDE F-18, SODIUM FLUORIDE F-18 3M * 3M CO PERIDEX, CHLORHEXIDINE GLUCONATE * 3M HEALTH CARE INC AVAGARD, ALCOHOL (OTC) DURAPREP, IODINE POVACRYLEX (OTC) 3M HEALTH CARE * 3M HEALTH CARE INFECTION PREVENTION DIV SOLUPREP, CHLORHEXIDINE GLUCONATE (OTC) ** 6 ** 60 DEGREES PHARMS * 60 DEGREES PHARMACEUTICALS LLC ARAKODA, TAFENOQUINE SUCCINATE ** A ** AAA USA INC * ADVANCED ACCELERATOR APPLICATIONS USA INC LUTATHERA, LUTETIUM DOTATATE LU-177 NETSPOT, GALLIUM DOTATATE GA-68 AAIPHARMA LLC * AAIPHARMA LLC AZASAN, AZATHIOPRINE ABBVIE * ABBVIE INC ANDROGEL, TESTOSTERONE CYCLOSPORINE, CYCLOSPORINE DEPAKOTE ER, DIVALPROEX SODIUM DEPAKOTE, DIVALPROEX SODIUM GENGRAF, CYCLOSPORINE K-TAB, POTASSIUM CHLORIDE KALETRA, LOPINAVIR NIASPAN, NIACIN NIMBEX PRESERVATIVE FREE, CISATRACURIUM BESYLATE NIMBEX, CISATRACURIUM BESYLATE NORVIR, RITONAVIR SYNTHROID, LEVOTHYROXINE SODIUM ** TARKA, TRANDOLAPRIL TRICOR, FENOFIBRATE TRILIPIX, CHOLINE FENOFIBRATE ULTANE, SEVOFLURANE ZEMPLAR, PARICALCITOL ABBVIE ENDOCRINE * ABBVIE ENDOCRINE INC LUPANETA PACK, LEUPROLIDE ACETATE ABBVIE ENDOCRINE INC * ABBVIE ENDOCRINE INC LUPRON DEPOT, LEUPROLIDE ACETATE LUPRON DEPOT-PED KIT, LEUPROLIDE ACETATE ABBVIE INC * ABBVIE INC DUOPA, CARBIDOPA MAVYRET, GLECAPREVIR NORVIR, RITONAVIR ORIAHNN (COPACKAGED), ELAGOLIX SODIUM,ESTRADIOL,NORETHINDRONE ACETATE -

NAFTIN (Naftifine Hydrochloride) Cream, 2%
HIGHLIGHTS OF PRESCRIBING INFORMATION ---------------------DOSAGE FORMS AND STRENGTHS--------------------- These highlights do not include all the information needed to use Cream: 2% (3) NAFTIN® Cream, 2% safely and effectively. See full prescribing information for NAFTIN (naftifine hydrochloride) Cream, 2%. -------------------------------CONTRAINDICATIONS----------------------------- None (4) NAFTIN (naftifine hydrochloride) cream, 2%, for topical use Initial U.S. Approval: 1988 -----------------------WARNINGS AND PRECAUTIONS-------------- Discontinue treatment if redness or irritation develops with NAFTIN Cream ---------------------------RECENT MAJOR CHANGES-------------------------- use. (5.1) Indications and Usage (1) 11/2016 ------------------------------ADVERSE REACTIONS------------------------------ ----------------------------INDICATIONS AND USAGE-------------------------- The most common adverse reaction (≥1%) is pruritus. (6.1) NAFTIN Cream is an allylamine antifungal indicated for the treatment of interdigital tinea pedis, tinea cruris, and tinea corporis caused by the organism To report SUSPECTED ADVERSE REACTIONS, contact Merz Trichophyton rubrum. (1) Pharmaceuticals, LLC at 877-743-8454 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. ----------------------DOSAGE AND ADMINISTRATION---------------------- For topical use only. NAFTIN Cream is not for ophthalmic, oral, or intravaginal use. (2) See 17 for PATIENT COUNSELING INFORMATION. Apply a thin layer of NAFTIN Cream once-daily to the affected areas plus a Revised: -

Walterreedarmy June2021idx 1..16
NCR-JOA UNIFIED MEDICATION FORMULARY Alphabetical Listing by Therapeutic Category This document is current as of Aug. 23, 2021 The availability of formulary items is subject to change. ABORTIFACIENT Abortifacient Ammonium Detoxicant MiFEPRIStone ........................................................................................23 Lactulose ................................................................................................ 20 Neomycin ................................................................................................24 Acetylcholinesterase Inhibitor Neostigmine ............................................................................................25 AMPA Glutamate Receptor Antagonist Pyridostigmine ........................................................................................29 Perampanel ............................................................................................ 27 Acetylcholinesterase Inhibitor (Central) Amylinomimetic Donepezil ................................................................................................12 Pramlintide ..............................................................................................28 Galantamine ........................................................................................... 16 Rivastigmine ...........................................................................................30 Analgesic Combination (Opioid) Acetaminophen and Codeine ...................................................................2 Acne -

Antifungals, Topical
EFFECTIVE 1/1/2021 Drug and Biologic Coverage Policy Effective Date ............................................ 1/1/2021 Next Review Date… ..................................... 1/1/2022 Coverage Policy Number ................................ P0046 Antifungals, Topical Table of Contents Related Coverage Resources Overview ...................................................................1 Coverage Policy Statement ......................................1 FDA Indication Criteria ..............................................2 Other Uses with Supportive Evidence Criteria .........3 Specific Additional Criteria ........................................3 Preferred Product Requirement Criteria ...................3 Conditions Not Covered............................................4 Background ...............................................................4 References ...............................................................5 INSTRUCTIONS FOR USE The following Coverage Policy applies to health benefit plans administered by Cigna Companies. Certain Cigna Companies and/or lines of business only provide utilization review services to clients and do not make coverage determinations. References to standard benefit plan language and coverage determinations do not apply to those clients. Coverage Policies are intended to provide guidance in interpreting certain standard benefit plans administered by Cigna Companies. Please note, the terms of a customer’s particular benefit plan document [Group Service Agreement, Evidence of Coverage, Certificate -

Investigations in Fish Control
INVESTIGATIONS IN FISH CONTROL 99. Evaluation of 215 Candidate Fungicides for Use in Fish Culture OCH 3 UNITED STATES DEPARTMENT OF THE INTERIOR FISH AND WILDLIFE SERVICE Investigations in Fish Control, published by the Fish and Wildlife Service, include reports on the results of work at the National Fisheries Research Center at LaCrosse, Wisconsin, and reports of other studies related to fish control and acute toxicity of piscicides. Though each report is regarded as a separate publication, several may be issued under a single cover, for economy. See inside back cover for list of current issues. Copies of this publication may be obtained from the Publications Unit, U.S. Fish and Wildlife Service, 18th and C Streets, NW., Mail Stop 1111, Room 130, Arlington Square Building, Washington, DC 20240, or may be purchased from the National Technical Information Service (NTIS), 5285 Port Royal Road, Springfield, VA 22161. ISSN 0565-0704 INVESTIGATIONS IN FISH CONTROL 99. Evaluation of 215 Candidate Fungicides for Use in Fish Culture By Tom A. Bailey and Susan M. Jeffrey U.S. Fish and Wildlife Service National Fisheries Research Center LaCrosse P.O. Box 818 LaCrosse, Wisconsin 54602 1989 Evaluation of 215 Candidate Fungicides for Use in Fish Culture by Tom A. Bailey and Susan M. Jeffrey U.S. Fish and Wildlife Service National Fisheries Research Center LaCrosse P.O. Box 818 LaCrosse, Wisconsin 54602 ABSTRACT. The fungicidal activity of 215 compounds was evaluated by comparing their effec tiveness with that of malachite green. More than half were found to be unsuitable as aquatic fungicides in preliminary screening tests because of their lack of activity against fungi, toxicity to fish or eggs, insolubility in water, or potential carcinogenicity.