Light Sources
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Technology Brief: Light Emitting Plasma
Energy Research & Development presents… Technology Brief: Light Emitting Plasma December 21, 2011 Report # ET11SMUD1018 Introduction • Emitter: the plasma lamp is housed within an aluminum By now, I’m certain most people in the lighting industry assembly specifically designed are familiar with LEDs – especially for outdoor lighting. to concentrate radio frequency But have you heard of Light Emitting Plasma? SMUD energy within the lamp. has been working with LUXIM Lighting and our customers to test this cutting edge technology. • Radio frequency (RF) Source: Luxim driver: connected to the Light Emitting Plasma (LEP) is a very intense, energy emitter via a coax cable and produces the efficient, white light source that could be used in a radio frequency energy needed to ignite and variety of outdoor lighting applications. operate the plasma lamp. What is Light Emitting Plasma? • AC/ DC power supply: converts incoming line voltage to DC and supplies power to the radio The Light Emitting Plasma system consists of the frequency driver. components listed below. These components are housed within a light fixture (a.k.a. luminaire). The Potential Benefits of Light Emitting Plasma luminaire also includes an optical assembly to distribute the light produced by the lamp. Light Emitting Plasma offers the following advantages over conventional high intensity discharge lamps: • Better reliability: conventional metal halide lamps require electrodes within the arc tube. These electrodes are usually made of tungsten and require a mechanical seal - which can lead to premature lamp failure. As the tungsten degrades, it darkens the walls of the lamp and reduces the light output. • Rapid start: 45 seconds to reach 80% of full brightness LEP System Components Source: LUXIM • Faster re-strike: when the power is turned off to high • Lamp: consists of a very small intensity discharge lamps Source: LUXIM quartz tube (3/4” long), which (e.g. -

Optical Measurements of Atmospheric Aerosols: Aeolian Dust, Secondary Organic Aerosols, and Laser-Induced Incandescence of Soot
Optical Measurements of Atmospheric Aerosols: Aeolian Dust, Secondary Organic Aerosols, and Laser-Induced Incandescence of Soot by Lulu Ma, B. S. A Dissertation In Chemistry Submitted to the Graduate Faculty of Texas Tech University in Partial Fulfillment of the Requirements for the Degree of Doctor of philosophy Approved Jonathan E. Thompson Chair of Committee Dimitri Pappas Carol L. Korzeniewski Dominick Casadonte Interim Dean of the Graduate School August, 2013 Copyright 2013, Lulu Ma Texas Tech University, Lulu Ma, August 2013 ACKNOWLEDGMENTS I would like to thank my advisor, Dr. Thompson for his guidance and encouragement throughout the past 4 years in Texas Tech University. His profound knowledge, diligence and patience encouraged me and has setup a great example for me in the future. I also thank to Dr. Pappas and Dr. Korzeniewski for their help and for taking time as my committee members. I also would like to thank Dr. Ted Zobeck for his help on soil dust measurements and soil sample collection and also Dr. P. Buseck and his lab for TEM analysis. My group members: Hao Tang, Fang Qian, Kathy Dial, Haley Redmond, Yiyi Wei, Tingting Cao, and Qing Zhang, also helped me a lot in both daily life, and research. At last, I would like to thank my parents and friends for their love and continuous encouragement and support. ii Texas Tech University, Lulu Ma, August 2013 TABLE OF CONTENTS ACKNOWLEDGMENTS……………………………………………………………….ii ABSTRACT ……………………………………………………………………………..vi LIST OF TABLES …………………………………………………………………….viii LIST OF FIGURES……………………………………………………..………………ix LIST OF ABBREVIATIONS ………………………………………….………………xi I. INTRODUCTION ......................................................................................................... 1 1.1 Introduction to Atmospheric Aerosols. ................................................................... 1 1.1.1 Natural Sources ............................................................................................. -

Commentary Kerosene-Based Lighting: an Overlooked Source of Exposure to Household Air Pollution?
Feature: Air quality challenges in low-income settlements Commentary Kerosene-based lighting: an overlooked source of exposure to household air pollution? Ariadna Curto 1,2,3, Cathryn Tonne 1,2,3 1Barcelona Institute for Global Health (ISGlobal), Barcelona, Spain 2Universitat Pompeu Fabra (UPF), Barcelona, Spain 3CIBER Epidemiología y Salud Pública (CIBERESP), Barcelona, Spain https://doi.org/10.17159/caj/2020/30/1.8420 Access to affordable, reliable, modern and sustainable energy is prolonged periods, resulting in high levels of inhaled pollutants one of the seventeen Sustainable Development Goals (SDG) set (i.e. more mass inhaled per mass emitted). An experimental by the United Nations for 2030. However, estimates indicate that study in Kenya estimated that night kiosk vendors can inhale progress towards that goal is not on track: 650 million people 1560 µg of fine particles per day emitted by kerosene lamps worldwide are estimated to remain without access to electricity alone (Apple et al., 2010). Relatively few studies have attempted in 2030 (IEA, IRENA, UNSD, WB, WHO, 2019). Nine out of ten of to quantify the contribution of kerosene-based lighting to these people will live in Sub-Saharan Africa (SSA), mostly in rural particle exposure in populations in SSA. We previously reported communities, which face barriers in terms of affordability and that kerosene-based lighting was the strongest determinant supply. of 24-h average and peak personal exposure to black carbon among women living in a semi-rural area of Mozambique (Curto Without access to affordable and reliable clean energy to et al., 2019). Women who used kerosene as the primary source meet daily cooking, lighting, and heating needs, individuals of lighting had 81% and 93% higher average and peak personal rely on inefficient fuels and technologies that give rise to black carbon exposure, respectively, than those using electricity. -
Rushlight Index 1980-2006
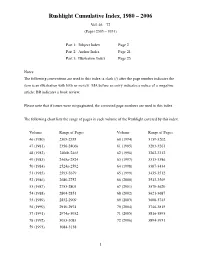
Rushlight Cumulative Index, 1980 – 2006 Vol. 46 – 72 (Pages 2305 – 3951) Part 1: Subject Index Page 2 Part 2: Author Index Page 21 Part 3: Illustration Index Page 25 Notes: The following conventions are used in this index: a slash (/) after the page number indicates the item is an illustration with little or no text. MA before an entry indicates a notice of a magazine article; BR indicates a book review. Please note that if issues were mispaginated, the corrected page numbers are used in this index. The following chart lists the range of pages in each volume of the Rushlight covered by this index. Volume Range of Pages Volume Range of Pages 46 (1980) 2305-2355 60 (1994) 3139-3202 47 (1981) 2356-2406a 61 (1995) 3203-3261 48 (1982) 2406b-2465 62 (1996) 3262-3312 49 (1983) 2465a-2524 63 (1997) 3313-3386 50 (1984) 2524a-2592 64 (1998) 3387-3434 51 (1985) 2593-2679 65 (1999) 3435-3512 52 (1986) 2680-2752 66 (2000) 3513-3569 53 (1987) 2753-2803 67 (2001) 3570-3620 54 (1988) 2804-2851 68 (2002) 3621-3687 55 (1989) 2852-2909 69 (2003) 3688-3745 56 (1990) 2910-2974 70 (2004) 3746-3815 57 (1991) 2974a-3032 71 (2005) 3816-3893 58 (1992) 3033-3083 72 (2006) 3894-3951 59 (1993) 3084-3138 1 Rushlight Subject Index Subject Page Andrews' burning fluid vapor lamps 3400-05 Abraham Gesner: Father of Kerosene 2543-47 Andrews patent vapor burner 3359/ Accessories for decorating lamps 2924 Andrews safety lamp, award refused 3774 Acetylene bicycle lamps, sandwich style 3071-79 Andrews, Solomon, 1831 gas generator 3401 Acetylene bicycle lamps, Solar 2993-3004 -

PLS(Plasma Lighting System)
Outdoor Floodlight, Indoor Light, Light Pipe, Image Pole can be applied for each usage. PLS Products Plasma Lighting System Flood Lighting PSF 1032A Indoor Lighting PSH 0731B Another natural light, the PLS The ideal lamp for the next generation, the PLS, with LG digital technology giving you a supreme lighting environment. P l a s m a L i g h t i n g S y s t e m Providing light from a non-electrode plasma lamp, the PLS! Almost like sunlight, with the full spectrum of natural white light, good for eyesight preservation without fluorescent substances, and comfortable and eco-friendly in any location with minimized Ultraviolet and Infrared radiation. Enjoy the finest natural lighting with the PLS. Why choose the PLS? Plasma Lighting System More Natural, More More Environmentally-Friendly Light Source Another Sun! The artificial light that can imitate sunlight the best provides the most pleasant lighting. Long lasting! Non-electrode technology makes it possible to preserve the light's initial brightness, for a longer product lifetime. High efficiency! Reduction in energy consumption and cost, with high luminous flux and lumen maintenance Optimal for Human Visual Sensitivity! Helps improve recognition of contrast and motion in the dark Eco-friendly! Contains no mercury, for a better environment and your better health PLS is... The PLS (Plasma Lighting System) is a new concept of lighting utilizing the principle of plasma light emission through microwaves. Its core aim is to provide a lighting sensation worldwide, using the new and different technology of the light source. LG Electronics have successfully paved the way to the commercialization of this PLS through their previous accomplishments with microwave-applied technology and persistent research. -

Technology Meets Art: the Wild & Wessel Lamp Factory in Berlin And
António Cota Fevereiro Technology Meets Art: The Wild & Wessel Lamp Factory in Berlin and the Wedgwood Entrepreneurial Model Nineteenth-Century Art Worldwide 19, no. 2 (Autumn 2020) Citation: António Cota Fevereiro, “Technology Meets Art: The Wild & Wessel Lamp Factory in Berlin and the Wedgwood Entrepreneurial Model,” Nineteenth-Century Art Worldwide 19, no. 2 (Autumn 2020), https://doi.org/10.29411/ncaw.2020.19.2.2. Published by: Association of Historians of Nineteenth-Century Art Notes: This PDF is provided for reference purposes only and may not contain all the functionality or features of the original, online publication. License: This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License Creative Commons License. Accessed: October 30 2020 Fevereiro: The Wild & Wessel Lamp Factory in Berlin and the Wedgwood Entrepreneurial Model Nineteenth-Century Art Worldwide 19, no. 2 (Autumn 2020) Technology Meets Art: The Wild & Wessel Lamp Factory in Berlin and the Wedgwood Entrepreneurial Model by António Cota Fevereiro Few domestic conveniences in the long nineteenth century experienced such rapid and constant transformation as lights. By the end of the eighteenth century, candles and traditional oil lamps—which had been in use since antiquity—began to be superseded by a new class of oil-burning lamps that, thanks to a series of improvements, provided considerably more light than any previous form of indoor lighting. Plant oils (Europe) or whale oil (United States) fueled these lamps until, by the middle of the nineteenth century, they were gradually replaced by a petroleum derivative called kerosene. Though kerosene lamps remained popular until well into the twentieth century (and in some places until today), by the late nineteenth century they began to be supplanted by gas and electrical lights. -

A High-Power Source of Optical Radiation with Microwave Excitation
ISBN: 978-84-9048-719-8 DOI: http://dx.doi.org/10.4995/Ampere2019.2019.9761 A HIGH-POWER SOURCE OF OPTICAL RADIATION WITH MICROWAVE EXCITATION G. Churyumov1, 2, A. Denisov1, T. Frolova2, N. Wang1, J. Qiu1 11Harbin Institute of Technology, 92, West Dazhi Street, Nan Gang District, Harbin, China 2 Kharkiv National University of Radio Electronics, 14, Nauky Ave., 61166, Kharkiv, Ukraine [email protected] Keywords: microwave heating, magnetron, electrodeless sulfur lamp, plasma, microwave excitation 1. Introduction For more than 50 years, interest to the microwave heating technology has not weakened. In addition to the traditional areas of its application, which described in detail in [1], recently there has been an expansion of technological possibilities for the use of microwave energy associated with the impact of electromagnetic waves of the microwave range on various materials (sintering of metal and ceramic powders) and media, including plasma [2]. One such new direction is the creation of high-power and environmentally friendly sources of optical radiation on the basis of the electrodeless sulfur lamp with microwave excitation [2, 3]. As it is known, Michael Ury and his associates at Fusion Systems invented this radically new lamp in 1990, but the lamp was not ideal because of the complexity of its design [4]. Therefore, it was not put into production. However, every year the scientific interest was growing. An analysis of scientific publications shows that every 5 years a new country is joined to this issue. Now more than in 10 countries of all would including USA, Great Britain, South Korea, Netherlands, Germany, Russia, and so on where there are research teams that are carried out an investigation concerning the electrodeless lamps with microwave excitation. -

Lamp Basics Compiled.Pdf
Lamp Basics Lost-in-Time Museum Lamp Fuel One of the earliest types of lamp fuel was animal tallow, usually rendered from beef or mutton fat. Normally solid at room temperatures, it had to be heated to stay liquid. Lamp designs began to emerge in the late 1700’s using lighter fluids, which could travel vertically up a wick and produce a brighter, more efficient light. Whale oil, already used in candle making, was found to be an excellent oil for this new type of lamp. As the market grew, prices for whale oil soared. Burning fluid and camphene, two substitutes for whale oil, were used as early as the 1830’s, but were dangerous due to their flammability. Their use became obsolete as kerosene became more affordable. Kerosene, first produced in 1846 by distilling cannel coal, was commonly referred to as ‘coal oil’. The word "Kerosene", a registered trademark in 1854, eventually became a generic name used by all refiners. While safer and cleaner than burning fluids and camphene, it was expensive and not affordable to the everyday consumer. Kerosene distilled from petroleum revolutionized the lamp fuel market. As production methods became more efficient in the 1860’s, this new type of kerosene, which produced a brighter and cleaner light, gradually became the fuel source used by the general populace. An improved distribution network for natural gas and electricity, along with the technological advancements they made possible, led to the end of kerosene as a primary fuel source. Kerosene is now mainly used for residential heating and as an additive for insecticides and aviation fuel. -

Tucson Lighting, 1882-1912, with Information on Lighting the Historic Interior
Tucson lighting, 1882-1912, with information on lighting the historic interior Item Type text; Thesis-Reproduction (electronic) Authors Orlando, Catherine Maier Publisher The University of Arizona. Rights Copyright © is held by the author. Digital access to this material is made possible by the University Libraries, University of Arizona. Further transmission, reproduction or presentation (such as public display or performance) of protected items is prohibited except with permission of the author. Download date 28/09/2021 00:48:28 Link to Item http://hdl.handle.net/10150/557708 TUCSON LIGHTING, 1882-1912, WITH INFORMATION ON LIGHTING THE HISTORIC INTERIOR by Catherine Maier Orlando A Thesis Submitted to the Faculty of the SCHOOL OF HOME ECONOMICS In Partial Fulfillment of the Requirements For the Degree of MASTER OF SCIENCE In the Graduate College THE UNIVERSITY OF ARIZONA 19 8 1 STATEMENT BY AUTHOR This thesis has been submitted in partial fulfill ment of requirements for an advanced degree at The Univer sity of Arizona and is deposited in the University Library to be made available to borrowers under rules of the Library. Brief quotations from this thesis are allowable without special permission, provided that accurate acknowl edgment of source is made. Requests for permission for extended quotation from or reproduction of this manuscript in whole or in part may be granted by the head of the major department or the Dean of the Graduate College when in his judgment the proposed use of the material is in the inter ests of scholarship. In all other instances, however, permission must be obtained from the author. -

Understanding the Economics Behind Off-Grid Lighting Products for Small Businesses in Kenya
UNDERSTANDING THE ECONOMICS BEHIND OFF-GRID LIGHTING PRODUCTS FOR SMALL BUSINESSES IN KENYA By Kristen Radecsky A Thesis Presented to The Faculty of Humboldt State University In Partial Fulfillment Of the Requirements for the Degree Master of Science In Environmental Systems: Energy, Environment, and Society Option May, 2009 UNDERSTANDING THE ECONOMICS BEHIND OFF-GRID LIGHTING PRODUCTS FOR SMALL BUSINESSES IN KENYA By Kristen Radecsky Approved by the Master's Thesis Committee: Dr. Arne Jacobson, Major Professor Date Dr. Charles Chamberlin, Committee Member Date Dr. Steven Hackett, Committee Member Date Dr. Christopher Dugaw, Graduate Coordinator Date Dr. Chris A. Hopper, Dean for Research and Graduate Studies Date ABSTRACT UNDERSTANDING THE ECONOMICS BEHIND OFF-GRID LIGHTING PRODUCTS FOR SMALL BUSINESSES IN KENYA Kristen Radecsky For illumination, many off-grid communities use lighting products such as candles, kerosene-fueled lamps, or dry cell battery-powered lights. Unfortunately, fuel- based and dry cell powered lighting can be expensive, a health hazard and often provides poor quality light. Manufacturers are currently designing rechargeable lighting products using LED technology as an alternative option for lower-income people. I developed a model to analyze the initial and life cycle costs of 19 off-grid lighting products. With the results, I make design recommendations for manufacturers. The analysis is based on product prices, laboratory measurements of product performance, and data about lighting cost and use patterns for small, off-grid businesses in Kenya. The field data were collected by Arne Jacobson, Maina Mumbi, Peter Johnstone and me during 2008. My results indicate that the economics of off-grid lighting using electric lamps depends on the charging mode. -

Lifetime and Reliability of Polymer Solar Cells A
LIFETIME AND RELIABILITY OF POLYMER SOLAR CELLS A DISSERTATION SUBMITTED TO THE DEPARTMENT OF MATERIALS SCIENCE AND ENGINEERING AND THE COMMITTEE ON GRADUATE STUDIES OF STANFORD UNIVERSITY IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY Craig Homer Peters November 2011 © 2011 by Craig H Peters. All Rights Reserved. Re-distributed by Stanford University under license with the author. This dissertation is online at: http://purl.stanford.edu/fs540ky3123 ii I certify that I have read this dissertation and that, in my opinion, it is fully adequate in scope and quality as a dissertation for the degree of Doctor of Philosophy. Michael McGehee, Primary Adviser I certify that I have read this dissertation and that, in my opinion, it is fully adequate in scope and quality as a dissertation for the degree of Doctor of Philosophy. Reinhold Dauskardt I certify that I have read this dissertation and that, in my opinion, it is fully adequate in scope and quality as a dissertation for the degree of Doctor of Philosophy. Alan Sellinger Approved for the Stanford University Committee on Graduate Studies. Patricia J. Gumport, Vice Provost Graduate Education This signature page was generated electronically upon submission of this dissertation in electronic format. An original signed hard copy of the signature page is on file in University Archives. iii Abstract The power conversion efficiency of organic photovoltaic (OPV) cells has increased from 4-5% in 2005 to 8.3% in 2010. The goal of a 10% single junction OPV device seems attainable making the commercialization of OPV more realistic. -

USB Plasma Ball Instruction Manual
English USB Plasma Ball Instruction Manual Features Small plasma ball for your desk Electric discharge creates an amazing optical spectacle Power supply via USB cable (included) or 5 V DC adaptor (not included) Reacts to touches on the glass Visible rays of light come together where the glass ball is touched On/Off switch Included in the delivery Plasma ball USB cable (length: 0.95 m) Instruction manual How to get started 1. Remove the USB Plasma Ball from the packaging. 2. Position the USB Plasma Ball on a flat, solid surface. 3. Connect the USB cable with a free USB port on your computer. 4. Switch on the USB Plasma Ball. 5. Touch the glass ball with your hand or a conducting metal item e.g. a metal spoon. The rays of light come together where the glass ball is touched. 6. Make a noise (e.g. clap, sing or shout) and watch the spectacle inside the USB Plasma Ball as flashes of light are created to the sound. 7. If not being used, switch off the USB Plasma Ball. 1 English USB Plasma Ball Instruction Manual Additional information about the Plasma Ball The Plasma Ball or Plasma Lamp goes back to an invention by the physicist and electrical engineer Nikola Tesla in 1904. He called this invention an inert gas discharge tube. The Plasma Ball as we know it today is often demonstrated in school, universities or museums. It is found as a decorative accessory in many homes, too. However, it still functions in the same manner as Tesla’s discharge tube.