Joyce Bentum.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ghana Gazette
GHANA GAZETTE Published by Authority CONTENTS PAGE Facility with Long Term Licence … … … … … … … … … … … … 1236 Facility with Provisional Licence … … … … … … … … … … … … 201 Page | 1 HEALTH FACILITIES WITH LONG TERM LICENCE AS AT 12/01/2021 (ACCORDING TO THE HEALTH INSTITUTIONS AND FACILITIES ACT 829, 2011) TYPE OF PRACTITIONER DATE OF DATE NO NAME OF FACILITY TYPE OF FACILITY LICENCE REGION TOWN DISTRICT IN-CHARGE ISSUE EXPIRY DR. THOMAS PRIMUS 1 A1 HOSPITAL PRIMARY HOSPITAL LONG TERM ASHANTI KUMASI KUMASI METROPOLITAN KPADENOU 19 June 2019 18 June 2022 PROF. JOSEPH WOAHEN 2 ACADEMY CLINIC LIMITED CLINIC LONG TERM ASHANTI ASOKORE MAMPONG KUMASI METROPOLITAN ACHEAMPONG 05 October 2018 04 October 2021 MADAM PAULINA 3 ADAB SAB MATERNITY HOME MATERNITY HOME LONG TERM ASHANTI BOHYEN KUMASI METRO NTOW SAKYIBEA 04 April 2018 03 April 2021 DR. BEN BLAY OFOSU- 4 ADIEBEBA HOSPITAL LIMITED PRIMARY HOSPITAL LONG-TERM ASHANTI ADIEBEBA KUMASI METROPOLITAN BARKO 07 August 2019 06 August 2022 5 ADOM MMROSO MATERNITY HOME HEALTH CENTRE LONG TERM ASHANTI BROFOYEDU-KENYASI KWABRE MR. FELIX ATANGA 23 August 2018 22 August 2021 DR. EMMANUEL 6 AFARI COMMUNITY HOSPITAL LIMITED PRIMARY HOSPITAL LONG TERM ASHANTI AFARI ATWIMA NWABIAGYA MENSAH OSEI 04 January 2019 03 January 2022 AFRICAN DIASPORA CLINIC & MATERNITY MADAM PATRICIA 7 HOME HEALTH CENTRE LONG TERM ASHANTI ABIREM NEWTOWN KWABRE DISTRICT IJEOMA OGU 08 March 2019 07 March 2022 DR. JAMES K. BARNIE- 8 AGA HEALTH FOUNDATION PRIMARY HOSPITAL LONG TERM ASHANTI OBUASI OBUASI MUNICIPAL ASENSO 30 July 2018 29 July 2021 DR. JOSEPH YAW 9 AGAPE MEDICAL CENTRE PRIMARY HOSPITAL LONG TERM ASHANTI EJISU EJISU JUABEN MUNICIPAL MANU 15 March 2019 14 March 2022 10 AHMADIYYA MUSLIM MISSION -ASOKORE PRIMARY HOSPITAL LONG TERM ASHANTI ASOKORE KUMASI METROPOLITAN 30 July 2018 29 July 2021 AHMADIYYA MUSLIM MISSION HOSPITAL- DR. -

FORESTRY COMMISSION of GHANA Stakeholder Engagement
FORESTRY COMMISSION OF GHANA MINISTRY OF LANDS AND NATURAL RESOURCES Republic of Ghana STRATEGIC ENVIRONMENTAL AND SOCIAL ASSESSMENT (SESA) FOR REDD+ MECHANISM IN GHANA Stakeholder Engagement Report (SESA Report Annex 4) Prepared by SAL Consult Ltd, P O Box GP20200, Accra, Ghana August 2016 SAL Consult Ltd Forestry Commission TABLE OF CONTENTS TABLE OF CONTENTS .............................................................................................................................................. II ACRONYMS AND ABBREVIATIONS ........................................................................................................................ III 1.0 INTRODUCTION AND SUMMARY OF CONCERNS AND ISSUES FROM THE CONSULTATIONS .......................... 1 1.1 INTRODUCTION .................................................................................................................................................... 1 1.2 SUMMARY OF CONCERNS AND ISSUES FROM THE INITIAL STAKEHOLDER CONSULTATIONS .................................................... 1 1.2.1 Political and Economic Issues ................................................................................................................. 1 1.2.2 Legal and Policy ..................................................................................................................................... 2 1.2.3 Environmental Issues ............................................................................................................................. 4 1.2.4 Socio-economic and cultural -

Ghana Wildlife Society
Stakeholder Engagement Plan 28th May 2017 CEPF Grant 100805 Ghana Wildlife Society Project Title: Mainstreaming Site-Scale Ecosystem Values into Local Decision-Making in Ghana Project Location: Cape Three Points, Tano Offin and Atiwa Range Forest Reserves Grant Summary 1. Grantee organization: Ghana Wildlife Society 2. Grant title: Mainstreaming Site-Scale Ecosystem Values into Local Decision-Making in Ghana 3. Grant number: 100805 4. Grant amount (US dollars): 99,328.35 5. Proposed dates of grant: 1st August, 2017 – 31st January, 2019 6. Countries or territories where project will be undertaken: Ghana. 7. Date of preparation of this document: 28th May, 2017 8. Introduction: Information on the physical changes to forest ecosystems and the socio-economic consequences that might result from alternative land uses i.e. the "costs and benefits" of those changes is not well developed among local government agencies, the Forestry Commission and private sector actors. Moreover, some decision-makers are more likely to support ecosystem conservation for socio-economic reasons, rather than for the intrinsic value of biodiversity and associated ethical reasons. Although some of the ecosystem services are well known to include food, fuel, fibre and provision of cultural services, that provide benefits to people through recreation and cultural appreciation of nature, other services provided by the three KBAs selected for the project are not so well known. These include the regulation of the climate, purification of air and water and flood protection, soil formation and nutrient cycling. Hence, the case for conservation can be made more compelling for the afore-mentioned audience if the relationship between biodiversity and ecosystem services and the value of ecosystem services provided by key biodiversity areas (sites) are better understood. -

Public Procurement Authority. Draft Entity Categorization List
PUBLIC PROCUREMENT AUTHORITY. DRAFT ENTITY CATEGORIZATION LIST A Special Constitutional Bodies Bank of Ghana Council of State Judicial Service Parliament B Independent Constitutional Bodies Commission on Human Rights and Administrative Justice Electoral Commission Ghana Audit Service Lands Commission Local Government Service Secretariat National Commission for Civic Education National Development Planning Commission National Media Commission Office of the Head of Civil Service Public Service Commission Veterans Association of Ghana Ministries Ministry for the Interior Ministry of Chieftaincy and Traditional Affairs Ministry of Communications Ministry of Defence Ministry of Education Ministry of Employment and Labour Relations Ministry of Environment, Science, Technology and Innovation Ministry of Finance Ministry Of Fisheries And Aquaculture Development Ministry of Food & Agriculture Ministry Of Foreign Affairs And Regional Integration Ministry of Gender, Children and Social protection Ministry of Health Ministry of Justice & Attorney General Ministry of Lands and Natural Resources Ministry of Local Government and Rural Development Ministry of Petroleum Ministry of Power PUBLIC PROCUREMENT AUTHORITY. DRAFT ENTITY CATEGORIZATION LIST Ministry of Roads and Highways Ministry of Tourism, Culture and Creative Arts Ministry of Trade and Industry Ministry of Transport Ministry of Water Resources, Works & Housing Ministry Of Youth And Sports Office of the President Office of President Regional Co-ordinating Council Ashanti - Regional Co-ordinating -

Chapter 3 Present Situation of Ghana, Ashanti Region and Greater Kumasi Sub-Region 3.1 Past Development Trend and Current Development Policies of Ghana
13-203 13-203 The Study on the Comprehensive Urban Development Plan for Greater Kumasi in the Republic of Ghana Final Report Table of Contents Volume 1 Page Study Area: Greater Kumasi Sub-Region ................................................................................. 1 Photos of Greater Kumasi Sub-Region ..................................................................................... 2 Brief on the Study Project ......................................................................................................... 3 Executive Summary .................................................................................................................. 4 Conclusion ............................................................................................................................... 14 Part I Introduction Chapter 1 Introduction 1.1 Background .................................................................................................................. 1-1 1.2 Goals of the Study Project ............................................................................................ 1-2 1.3 Objectives of the Study Project .................................................................................... 1-2 1.4 Study Area .................................................................................................................... 1-3 1.5 Executive Agency, Implementing Agency and Other Counterpart Agencies ............... 1-3 1.6 Phases of the Project ................................................................................................... -
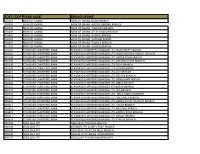
Sort Code Bank Name Branch Name
SORT CODE BANK NAME BRANCH NAME 010101 BANK OF GHANA BANK OF GHANA ACCRA BRANCH 010303 BANK OF GHANA BANK OF GHANA -AGONA SWEDRU BRANCH 010401 BANK OF GHANA BANK OF GHANA -TAKORADI BRANCH 010402 BANK OF GHANA BANK OF GHANA -SEFWI BOAKO BRANCH 010601 BANK OF GHANA BANK OF GHANA -KUMASI BRANCH 010701 BANK OF GHANA BANK OF GHANA -SUNYANI BRANCH 010801 BANK OF GHANA BANK OF GHANA -TAMALE BRANCH 011101 BANK OF GHANA BANK OF GHANA - HOHOE BRANCH 020101 STANDARD CHARTERED BANK STANDARD CHARTERED BANK(GH) LTD-HIGH STREET BRANCH 020102 STANDARD CHARTERED BANK STANDARD CHARTERED BANK(GH) LTD- INDEPENDENCE AVENUE BRANCH 020104 STANDARD CHARTERED BANK STANDARD CHARTERED BANK(GH) LTD-LIBERIA ROAD BRANCH 020105 STANDARD CHARTERED BANK STANDARD CHARTERED BANK(GH) LTD-OPEIBEA HOUSE BRANCH 020106 STANDARD CHARTERED BANK STANDARD CHARTERED BANK(GH) LTD-TEMA BRANCH 020108 STANDARD CHARTERED BANK STANDARD CHARTERED BANK(GH) LTD-LEGON BRANCH 020112 STANDARD CHARTERED BANK STANDARD CHARTERED BANK(GH) LTD-OSU BRANCH 020118 STANDARD CHARTERED BANK STANDARD CHARTERED BANK(GH) LTD-SPINTEX BRANCH 020121 STANDARD CHARTERED BANK STANDARD CHARTERED BANK(GH) LTD-DANSOMAN BRANCH 020126 STANDARD CHARTERED BANK STANDARD CHARTERED BANK(GH) LTD-ABEKA BRANCH 020127 STANDARD CHARTERED BANK STANDARD CHARTERED BANK(GH)-ACHIMOTA BRANCH 020129 STANDARD CHARTERED BANK STANDARD CHARTERED BANK(GH) LTD- NIA BRANCH 020132 STANDARD CHARTERED BANK STANDARD CHARTERED BANK(GH) LTD- TEMA HABOUR BRANCH 020133 STANDARD CHARTERED BANK STANDARD CHARTERED BANK(GH) LTD- WESTHILLS BRANCH 020436 -

Ashanti Region
REGIONAL ANALYTICAL REPORT ASHANTI REGION Ghana Statistical Service June, 2013 Copyright © 2013 Ghana Statistical Service Prepared by: Victor K. Nyanteng Peter Takyi Peprah Lawford Boateng Acheamfuor Emmanuel Nii Okang Tawiah Edited by: Samuel K. Gaisie Chief Editor: Nii Bentsi-Enchill ii PREFACE AND ACKNOWLEDGEMENT There cannot be any meaningful developmental activity without taking into account the characteristics of the population for whom the activity is targeted. The size of the population and its spatial distribution, growth and change over time, and socio-economic characteristics are all important in development planning. The Kilimanjaro Programme of Action on Population adopted by African countries in 1984 stressed the need for population to be considered as a key factor in the formulation of development strategies and plans. A population census is the most important source of data on the population in a country. It provides information on the size, composition, growth and distribution of the population at the national and sub-national levels. Data from the 2010 Population and Housing Census (PHC) will serve as reference for equitable distribution of resources, government services and the allocation of government funds among various regions and districts for education, health and other social services. The Ghana Statistical Service (GSS) is delighted to provide data users with an analytical report on the 2010 PHC at the regional level to facilitate planning and decision-making. This follows the publication of the National Analytical Report in May, 2013 which contained information on the 2010 PHC at the national level with regional comparisons. Conclusions and recommendations from these reports are expected to serve as a basis for improving the quality of life of Ghanaians through evidence-based policy formulation, planning, monitoring and evaluation of developmental goals and intervention programs. -

Journal of Arts & Humanities
Journal of Arts & Humanities Volume 08, Issue 10, 2019: 60-72 Article Received: 20-08-2019 Accepted: 04-09-2019 Available Online: 27-10-2019 ISSN: 2167-9045 (Print), 2167-9053 (Online) DOI: http://dx.doi.org/10.18533/journal.v8i10.1731 Semantically Ambiguous: An Overview of Some Akan and Dangmbe Riddles Jonathan Edward Tetteh Kuwornu-Adjaottor1, George Appiah2, Melvin Nartey3 ABSTRACT Riddles, like folktales are considered an important aspect of speech games that promote critical thinking. Due to the influx of foreign games, local riddles have recently lost their values. But as an important aspect of Akan and Dangme cultures, riddles are included in school curricula, especially at the higher learning institutions, to create awareness of their socio-cultural importance in the society and to the youth. The importance of riddles and brain teasers cannot be overemphasized. Policy makers are keenly interested in promoting the use of riddles as a means of empowering the Ghanaian youth with the capacity to resolve the growing challenges of our economy. This paper seeks to address some of the teething problems associated with some of the popular Akan and Dangme riddles. Using aspects of the ethnography of communication theory (Gumperz, 1972) and (Saville-Troike 2003), selected riddles from the two languages, collected from communities were analyzed descriptively by means of feature/componential method. The findings are that, the meanings and/or ideas they express are not certain due to the sameness of features between the items with which the riddles are framed and other items. The paper has thus added to literature in the discipline of semantics. -

The Office of the Head of Local Government Service
REPUBLIC OF GHANA THE OFFICE OF THE HEAD OF LOCAL GOVERNMENT SERVICE MEDIUM TERM EXPENDITURE FRAMEWORK (MTEF) FOR 2017-2019 2017 BUDGET ESTIMATES For copies of the LGS MTEF PBB Estimates, please contact the Public Relations Office of the Ministry: Ministry of Finance Public Relations Office New Building, Ground Floor, Room 001/ 003 P. O. Box MB 40, Accra – Ghana The LGS MTEF PBB Estimate for 2017 is also available on the internet at: www.mofep.gov.gh Local Government Service Page ii Table of Contents PART A: STRATEGIC OVERVIEW OF THE OFFICE OF THE HEAD OF LOCAL GOVERNMENT SERVICE (OHLGS) .......................................................................................1 1. GSGDA II POLICY OBJECTIVES .............................................................................. 1 2. GOAL .................................................................................................................. 1 3. CORE FUNCTIONS ............................................................................................... 1 4. POLICY OUTCOME INDICATORS AND TARGETS .................................................... 2 5. EXPENDITURE TRENDS ......................................................................................... 3 6. KEY ACHIEVEMENTS FOR 2016 ............................................................................ 4 PART B: BUDGET PROGRAM SUMMARY ...........................................................................5 PROGRAM 1: MANAGEMENT AND ADMINISTRATION .................................................. 5 PROGRAMME -

Manufacturing Capabilities in Ghana's Districts
Manufacturing capabilities in Ghana’s districts A guidebook for “One District One Factory” James Dzansi David Lagakos Isaac Otoo Henry Telli Cynthia Zindam May 2018 When citing this publication please use the title and the following reference number: F-33420-GHA-1 About the Authors James Dzansi is a Country Economist at the International Growth Centre (IGC), Ghana. He works with researchers and policymakers to promote evidence-based policy. Before joining the IGC, James worked for the UK’s Department of Energy and Climate Change, where he led several analyses to inform UK energy policy. Previously, he served as a lecturer at the Jonkoping International Business School. His research interests are in development economics, corporate governance, energy economics, and energy policy. James holds a PhD, MSc, and BA in economics and LLM in petroleum taxation and finance. David Lagakos is an associate professor of economics at the University of California San Diego (UCSD). He received his PhD in economics from UCLA. He is also the lead academic for IGC-Ghana. He has previously held positions at the Federal Reserve Bank of Minneapolis as well as Arizona State University, and is currently a research associate with the Economic Fluctuations and Growth Group at the National Bureau of Economic Research. His research focuses on macroeconomic and growth theory. Much of his recent work examines productivity, particularly as it relates to agriculture and developing economies, as well as human capital. Isaac Otoo is a research assistant who works with the team in Ghana. He has an MPhil (Economics) from the University of Ghana and his thesis/dissertation tittle was “Fiscal Decentralization and Efficiency of the Local Government in Ghana.” He has an interest in issues concerning local government and efficiency. -

FORESTRY COMMISSION TINTE BEPO FOREST RESERVE MANAGEMENT PLAN ( Mankranso Forest District)
FORESTRY COMMISSION TINTE BEPO FOREST RESERVE MANAGEMENT PLAN ( Mankranso Forest District) MARCH,2016 FOREWORD The constitution of each forest reserve mandated the then Forestry Department to develop working plans to be able to effectively and efficiently manage, protect and develop forest and wildlife resources in each of these reserves to benefit all segments of society. Nevertheless, a century of evolution of Forest Policy formulation, institutional transformation and management, including development and protection has given birth to a trendy and sophisticated societal demand on the forests of Ghana. These developments require the reinvigoration and revision of the existing working plans which were written, taking into consideration the socio-economic and cultural issues prevailing at that time. The policy and the legal framework which prevailed at the time of the constitution of these reserves emphasized on the contribution of forestry to environmental management inter alia the protection of water bodies and their sources, providing the necessary environment for our agricultural practices, prevention of erosion in mountainous areas and preservation of bio-diversity. This framework engendered the then Forestry Department to a non-participatory approach to resource management culminating in policing and less regard to the stakeholders. Over a period, forest and wildlife resources management regimes have undergone substantial changes in conformity with modern developmental trends, more especially international conventions and ramifications. -

Characterization of Solid Waste in the Atwima-Nwabiagya District of the Ashanti Region, Kumasi-Ghana
International Journal of Waste Management and Technology Vol. 2, No. 1, January 2014, PP: 1 - 14, ISSN: 2327-8757 (Online) Available online at www.ijwmt.com Research article CHARACTERIZATION OF SOLID WASTE IN THE ATWIMA-NWABIAGYA DISTRICT OF THE ASHANTI REGION, KUMASI-GHANA Philip Osei-Mensah1, Albert Amatey Adjaottor2, *Godfred Owusu-Boateng3 1, 2Department of Material Engineering, Kwame Nkrumah University of Science and Technology (KNUST), Kumasi, Ghana 3Faculty of Renewable Natural Resources, (KNUST), Kumasi, Ghana *Corresponding Author: [email protected] ______________________________________________________________________________ ABSTRACT Solid waste characterization study is fundamental to any proper planning of solid waste management in an area. This study was undertaken to assess the characteristics of the waste generated in the Atwima Nwabiagya District, of Ghana to enhance scientific management of solid wastes in the district. The Direct Waste Sorting was applied to solid wastes collected from fifty-three (53) households with different socio-economic characteristics over a two- week period. Results indicated that the average solid waste generation rate for the district was 0.66 kg/capita/day with a mean daily weight of 109000 kg, expected to grow by 34% by the year 2018. Solid wastes which were biodegradable and recyclable nature were dominated by food, yard and garden waste (54.8 %), plastics (3.1%), metals (0.3%), paper (1.6%), wood (0.3%), glass (0.2%), textiles (0.7%), sand and ash (40%) suggesting that an integrated waste management approach supported by willingness to source-separate wastes could be the best option for the district. With the right knowledge of the general culture of the communities and willingness to source- separate such approach would provide opportunity for the district to maximize revenue through compost preparation, recycling and land reclamation while reducing public health problems associated with management of solid waste.