Planetary Systems and the Origins of Life
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Planetas Extrasolares
Local Extrasolar Planets Universidad Andres Bello ESO Vitacura 4 June 2015 Foto: Dante Joyce Minniti Pullen The big picture 1. Increase human resources and networks for national Astronomy 2. Develop new areas of research and do quality science 3. Promote science in Chile Universidad Andres Bello ESO Vitacura 4 June 2015 Dante Minniti The Pioneers Wolfgang Gieren Maria Teresa Ruiz Grzegorz Pietrzynski Dante Minniti School on Extrasolar Planets and Brown Dwarfs Santiago, 2003 Invited Lecturers: Michel Mayor, Scott Tremaine, Gill Knapp, France Allard Universidad Andres Bello ESO Vitacura 4 June 2015 Dante Minniti It is all about time... Telescope time is the most precious... Universidad Andres Bello ESO Vitacura 4 June 2015 Dante Minniti The Pioneers Wolfgang Gieren Maria Teresa Ruiz Grzegorz Pietrzynski Dante Minniti The first exoplanet for us: M. Konacki, G. Torres, D. D. Sasselov, G. Pietrzynski, A. Udalski, S. Jha, M. T. Ruiz, W. Gieren, & D. Minniti, “A Transiting Extrasolar Giant Planet Around the Star OGLE-TR-113'', 2004, ApJ, 609, L37” Universidad Andres Bello ESO Vitacura 4 June 2015 Dante Minniti The Pioneers Paul Butler Debra Fischer Dante Minniti The First Planets from the N2K Consortium Fischer et al., ``A Hot Saturn Planet Orbiting HD 88133, from the N2K Consortium", 2005, The Astrophysical Journal, 620, 481 Sato, et al., ``The N2K Consortium. II. A Transiting Hot Saturn around HD 149026 with a Large Dense Core", 2005, The Astrophysical Journal, 633, 465 Universidad Andres Bello ESO Vitacura 4 June 2015 Dante Minniti The Pioneers -

Lurking in the Shadows: Wide-Separation Gas Giants As Tracers of Planet Formation
Lurking in the Shadows: Wide-Separation Gas Giants as Tracers of Planet Formation Thesis by Marta Levesque Bryan In Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy CALIFORNIA INSTITUTE OF TECHNOLOGY Pasadena, California 2018 Defended May 1, 2018 ii © 2018 Marta Levesque Bryan ORCID: [0000-0002-6076-5967] All rights reserved iii ACKNOWLEDGEMENTS First and foremost I would like to thank Heather Knutson, who I had the great privilege of working with as my thesis advisor. Her encouragement, guidance, and perspective helped me navigate many a challenging problem, and my conversations with her were a consistent source of positivity and learning throughout my time at Caltech. I leave graduate school a better scientist and person for having her as a role model. Heather fostered a wonderfully positive and supportive environment for her students, giving us the space to explore and grow - I could not have asked for a better advisor or research experience. I would also like to thank Konstantin Batygin for enthusiastic and illuminating discussions that always left me more excited to explore the result at hand. Thank you as well to Dimitri Mawet for providing both expertise and contagious optimism for some of my latest direct imaging endeavors. Thank you to the rest of my thesis committee, namely Geoff Blake, Evan Kirby, and Chuck Steidel for their support, helpful conversations, and insightful questions. I am grateful to have had the opportunity to collaborate with Brendan Bowler. His talk at Caltech my second year of graduate school introduced me to an unexpected population of massive wide-separation planetary-mass companions, and lead to a long-running collaboration from which several of my thesis projects were born. -

Miniature Exoplanet Radial Velocity Array I: Design, Commissioning, and Early Photometric Results
Miniature Exoplanet Radial Velocity Array I: design, commissioning, and early photometric results Jonathan J. Swift Steven R. Gibson Michael Bottom Brian Lin John A. Johnson Ming Zhao Jason T. Wright Paul Gardner Nate McCrady Emilio Falco Robert A. Wittenmyer Stephen Criswell Peter Plavchan Chantanelle Nava Reed Riddle Connor Robinson Philip S. Muirhead David H. Sliski Erich Herzig Richard Hedrick Justin Myles Kevin Ivarsen Cullen H. Blake Annie Hjelstrom Jason Eastman Jon de Vera Thomas G. Beatty Andrew Szentgyorgyi Stuart I. Barnes Downloaded From: http://astronomicaltelescopes.spiedigitallibrary.org/ on 05/21/2017 Terms of Use: http://spiedigitallibrary.org/ss/termsofuse.aspx Journal of Astronomical Telescopes, Instruments, and Systems 1(2), 027002 (Apr–Jun 2015) Miniature Exoplanet Radial Velocity Array I: design, commissioning, and early photometric results Jonathan J. Swift,a,*,† Michael Bottom,a John A. Johnson,b Jason T. Wright,c Nate McCrady,d Robert A. Wittenmyer,e Peter Plavchan,f Reed Riddle,a Philip S. Muirhead,g Erich Herzig,a Justin Myles,h Cullen H. Blake,i Jason Eastman,b Thomas G. Beatty,c Stuart I. Barnes,j,‡ Steven R. Gibson,k,§ Brian Lin,a Ming Zhao,c Paul Gardner,a Emilio Falco,l Stephen Criswell,l Chantanelle Nava,d Connor Robinson,d David H. Sliski,i Richard Hedrick,m Kevin Ivarsen,m Annie Hjelstrom,n Jon de Vera,n and Andrew Szentgyorgyil aCalifornia Institute of Technology, Departments of Astronomy and Planetary Science, 1200 E. California Boulevard, Pasadena, California 91125, United States bHarvard-Smithsonian Center for Astrophysics, Cambridge, Massachusetts 02138, United States cThe Pennsylvania State University, Department of Astronomy and Astrophysics, Center for Exoplanets and Habitable Worlds, 525 Davey Laboratory, University Park, Pennsylvania 16802, United States dUniversity of Montana, Department of Physics and Astronomy, 32 Campus Drive, No. -

The Search for Extrasolar Planets
zucker 16-12-2005 11:22 Pagina 229 229 The Search for Extrasolar Planets S. Zucker and M. Mayor Observatoire de Genève, Sauverny, Switzerland During the recent decade, the question of the existence of planets orbiting stars other than our Sun has been answered unequivocally. About 150 extrasolar plan- ets have been detected since 1995, and their properties are the subject of wide interest in the research community. Planet formation and evolution theories are adjusting to the constantly emerging data, and astronomers are seeking new ways to widen the sample and enrich the data about the known planets. In September 2002, ISSI organized a workshop focusing on the physics of “Planetary Systems and Planets in Systems”1. The present contribution is an attempt to give a broader overview of the researches in the field of exoplanets and results obtained in the decade after the discovery of the planet 51 Peg b. The existence of planets orbiting other stars was speculated upon even in the 4th century BC, when Epicurus and Aristotle debated it using their early notions about our world. Epicurus claimed that the infinity of the Universe compelled the existence of other worlds. After the Copernican Revolution, Giordano Bruno wrote: “Innumerable suns exist; innumerable earths revolve around these suns in a manner similar to the way the seven planets revolve around our Sun”. Aitken2 examined the observational problem of detecting extrasolar planets. He showed that their detection, either directly or indirectly, lay beyond the techni- cal horizon of his era. The basic difficulty in directly detecting planets lies in the brightness ratio between a typical planet and its host star, a ratio that can be as low as 10-8. -

9905625.PDF (3.665Mb)
INFORMATION TO USERS This manuscript has been reproduced from the microfilm master. UMI films the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are in typewriter free, while others may be from any type o f computer printer. The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleedthrough, substandard margins, and improper alignment can adversely afreet reproduction. In the unlikely event that the author did not send UMI a complete manuscript and there are m is^ g pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note will indicate the deletion. Oversize materials (e.g., m ^ s, drawings, charts) are reproduced by sectioning the orignal, begnning at the upper left-hand comer and continuing from left to right in equal sections with small overlaps. Each original is also photographed in one exposure and is included in reduced form at the back of the book. Photographs included in the original manuscript have been reproduced xerographically in this copy. KGgher quality 6” x 9” black and white photographic prints are available for any photographs or illustrations appearing in this copy for an additional charge. Contact UMI directly to order. UMI A Bell & Howell Infoimation Compaiy 300 North Zeeb Road, Ann Arbor MI 48106-1346 USA 313/761-4700 800/521-0600 UNIVERSITY OF OKLAHOMA GRADUATE COLLEGE “ ‘TIS SOMETHING, NOTHING”, A SEARCH FOR RADIO SUPERNOVAE A DISSERTATION SUBMITTED TO THE GRADUATE FACULTY in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY By CHRISTOPHER R. -
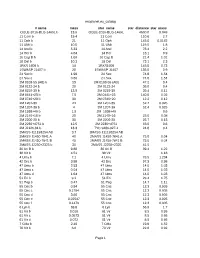
Exoplanet.Eu Catalog Page 1 # Name Mass Star Name
exoplanet.eu_catalog # name mass star_name star_distance star_mass OGLE-2016-BLG-1469L b 13.6 OGLE-2016-BLG-1469L 4500.0 0.048 11 Com b 19.4 11 Com 110.6 2.7 11 Oph b 21 11 Oph 145.0 0.0162 11 UMi b 10.5 11 UMi 119.5 1.8 14 And b 5.33 14 And 76.4 2.2 14 Her b 4.64 14 Her 18.1 0.9 16 Cyg B b 1.68 16 Cyg B 21.4 1.01 18 Del b 10.3 18 Del 73.1 2.3 1RXS 1609 b 14 1RXS1609 145.0 0.73 1SWASP J1407 b 20 1SWASP J1407 133.0 0.9 24 Sex b 1.99 24 Sex 74.8 1.54 24 Sex c 0.86 24 Sex 74.8 1.54 2M 0103-55 (AB) b 13 2M 0103-55 (AB) 47.2 0.4 2M 0122-24 b 20 2M 0122-24 36.0 0.4 2M 0219-39 b 13.9 2M 0219-39 39.4 0.11 2M 0441+23 b 7.5 2M 0441+23 140.0 0.02 2M 0746+20 b 30 2M 0746+20 12.2 0.12 2M 1207-39 24 2M 1207-39 52.4 0.025 2M 1207-39 b 4 2M 1207-39 52.4 0.025 2M 1938+46 b 1.9 2M 1938+46 0.6 2M 2140+16 b 20 2M 2140+16 25.0 0.08 2M 2206-20 b 30 2M 2206-20 26.7 0.13 2M 2236+4751 b 12.5 2M 2236+4751 63.0 0.6 2M J2126-81 b 13.3 TYC 9486-927-1 24.8 0.4 2MASS J11193254 AB 3.7 2MASS J11193254 AB 2MASS J1450-7841 A 40 2MASS J1450-7841 A 75.0 0.04 2MASS J1450-7841 B 40 2MASS J1450-7841 B 75.0 0.04 2MASS J2250+2325 b 30 2MASS J2250+2325 41.5 30 Ari B b 9.88 30 Ari B 39.4 1.22 38 Vir b 4.51 38 Vir 1.18 4 Uma b 7.1 4 Uma 78.5 1.234 42 Dra b 3.88 42 Dra 97.3 0.98 47 Uma b 2.53 47 Uma 14.0 1.03 47 Uma c 0.54 47 Uma 14.0 1.03 47 Uma d 1.64 47 Uma 14.0 1.03 51 Eri b 9.1 51 Eri 29.4 1.75 51 Peg b 0.47 51 Peg 14.7 1.11 55 Cnc b 0.84 55 Cnc 12.3 0.905 55 Cnc c 0.1784 55 Cnc 12.3 0.905 55 Cnc d 3.86 55 Cnc 12.3 0.905 55 Cnc e 0.02547 55 Cnc 12.3 0.905 55 Cnc f 0.1479 55 -

Report by the ESA–ESO Working Group on Extra-Solar Planets
Report by the ESA–ESO Working Group on Extra-Solar Planets 4 March 2005 Summary Various techniques are being used to search for extra-solar planetary signatures, including accurate measurement of radial velocity and positional (astrometric) dis- placements, gravitational microlensing, and photometric transits. Planned space experiments promise a considerable increase in the detections and statistical know- ledge arising especially from transit and astrometric measurements over the years 2005–15, with some hundreds of terrestrial-type planets expected from transit mea- surements, and many thousands of Jupiter-mass planets expected from astrometric measurements. Beyond 2015, very ambitious space (Darwin/TPF) and ground (OWL) experiments are targeting direct detection of nearby Earth-mass planets in the habitable zone and the measurement of their spectral characteristics. Beyond these, ‘Life Finder’ (aiming to produce confirmatory evidence of the presence of life) and ‘Earth Imager’ (some massive interferometric array providing resolved images of a distant Earth) arXiv:astro-ph/0506163v1 8 Jun 2005 appear as distant visions. This report, to ESA and ESO, summarises the direction of exo-planet research that can be expected over the next 10 years or so, identifies the roles of the major facilities of the two organisations in the field, and concludes with some recommendations which may assist development of the field. The report has been compiled by the Working Group members and experts (page iii) over the period June–December 2004. Introduction & Background Following an agreement to cooperate on science planning issues, the executives of the European Southern Observatory (ESO) and the European Space Agency (ESA) Science Programme and representatives of their science advisory structures have met to share information and to identify potential synergies within their future projects. -

A Search for Variability and Transit Signatures In
A SEARCH FOR VARIABILITY AND TRANSIT SIGNATURES IN HIPPARCOS PHOTOMETRIC DATA A thesis presented to the faculty of 3 ^ San Francisco State University Zo\% In partial fulfilment of W* The Requirements for The Degree Master of Science In Physics: Astronomy by Badrinath Thirumalachari San JVancisco, California December 2018 Copyright by Badrinath Thirumalachari 2018 CERTIFICATION OF APPROVAL I certify that I have read A SEARCH FOR VARIABILITY AND TRANSIT SIGNATURES IN HIPPARCOS PHOTOMETRIC DATA by Badrinath Thirumalachari and that in my opinion this work meets the criteria for approving a thesis submitted in partial fulfillment of the requirements for the degree: Master of Science in Physics: Astronomy at San Francisco State University. fov- Dr. Stephen Kane, Ph.D. Astrophysics Associate Professor of Planetary Astrophysics Dr. Uo&eph Barranco, Ph.D. .%trtJphysics Chairfe Associate Professor of Physics K + A Q , L a . Dr. Ron Marzke, Ph.D. Astronomy Assoc. Dean of College of Science & Engineering A SEARCH FOR VARIABILITY AND TRANSIT SIGNATURES IN HIPPARCOS PHOTOMETRIC DATA Badrinath Thirumalachari San Francisco State University 2018 The study and characterization of exoplanets has picked up pace rapidly over the past few decades with the invention of newer techniques and instruments. Detecting transits in stellar photometric data around stars already known to harbor exoplanets is crucial for exoplanet characterization. Due to these advancements we now have oceans of data and coming up with an automated way of performing exoplanet characterization is a challenge. In this thesis I describe one such method to search for transits in Hipparcos dataset containing photometric data for over 118000 stars. The radial velocity method has discovered a lot of planets around bright host stars and a follow up transit detection will give us the density of the exoplanet. -

Mètodes De Detecció I Anàlisi D'exoplanetes
MÈTODES DE DETECCIÓ I ANÀLISI D’EXOPLANETES Rubén Soussé Villa 2n de Batxillerat Tutora: Dolors Romero IES XXV Olimpíada 13/1/2011 Mètodes de detecció i anàlisi d’exoplanetes . Índex - Introducció ............................................................................................. 5 [ Marc Teòric ] 1. L’Univers ............................................................................................... 6 1.1 Les estrelles .................................................................................. 6 1.1.1 Vida de les estrelles .............................................................. 7 1.1.2 Classes espectrals .................................................................9 1.1.3 Magnitud ........................................................................... 9 1.2 Sistemes planetaris: El Sistema Solar .............................................. 10 1.2.1 Formació ......................................................................... 11 1.2.2 Planetes .......................................................................... 13 2. Planetes extrasolars ............................................................................ 19 2.1 Denominació .............................................................................. 19 2.2 Història dels exoplanetes .............................................................. 20 2.3 Mètodes per detectar-los i saber-ne les característiques ..................... 26 2.3.1 Oscil·lació Doppler ........................................................... 27 2.3.2 Trànsits -

Search for Brown-Dwarf Companions of Stars⋆⋆⋆
A&A 525, A95 (2011) Astronomy DOI: 10.1051/0004-6361/201015427 & c ESO 2010 Astrophysics Search for brown-dwarf companions of stars, J. Sahlmann1,2, D. Ségransan1,D.Queloz1,S.Udry1,N.C.Santos3,4, M. Marmier1,M.Mayor1, D. Naef1,F.Pepe1, and S. Zucker5 1 Observatoire de Genève, Université de Genève, 51 Chemin des Maillettes, 1290 Sauverny, Switzerland e-mail: [email protected] 2 European Southern Observatory, Karl-Schwarzschild-Str. 2, 85748 Garching bei München, Germany 3 Centro de Astrofísica, Universidade do Porto, Rua das Estrelas, 4150-762 Porto, Portugal 4 Departamento de Física e Astronomia, Faculdade de Ciências, Universidade do Porto, Portugal 5 Department of Geophysics and Planetary Sciences, Tel Aviv University, Tel Aviv 69978, Israel Received 19 July 2010 / Accepted 23 September 2010 ABSTRACT Context. The frequency of brown-dwarf companions in close orbit around Sun-like stars is low compared to the frequency of plane- tary and stellar companions. There is presently no comprehensive explanation of this lack of brown-dwarf companions. Aims. By combining the orbital solutions obtained from stellar radial-velocity curves and Hipparcos astrometric measurements, we attempt to determine the orbit inclinations and therefore the masses of the orbiting companions. By determining the masses of poten- tial brown-dwarf companions, we improve our knowledge of the companion mass-function. Methods. The radial-velocity solutions revealing potential brown-dwarf companions are obtained for stars from the CORALIE and HARPS planet-search surveys or from the literature. The best Keplerian fit to our radial-velocity measurements is found using the Levenberg-Marquardt method. -

Abstracts of Extreme Solar Systems 4 (Reykjavik, Iceland)
Abstracts of Extreme Solar Systems 4 (Reykjavik, Iceland) American Astronomical Society August, 2019 100 — New Discoveries scope (JWST), as well as other large ground-based and space-based telescopes coming online in the next 100.01 — Review of TESS’s First Year Survey and two decades. Future Plans The status of the TESS mission as it completes its first year of survey operations in July 2019 will bere- George Ricker1 viewed. The opportunities enabled by TESS’s unique 1 Kavli Institute, MIT (Cambridge, Massachusetts, United States) lunar-resonant orbit for an extended mission lasting more than a decade will also be presented. Successfully launched in April 2018, NASA’s Tran- siting Exoplanet Survey Satellite (TESS) is well on its way to discovering thousands of exoplanets in orbit 100.02 — The Gemini Planet Imager Exoplanet Sur- around the brightest stars in the sky. During its ini- vey: Giant Planet and Brown Dwarf Demographics tial two-year survey mission, TESS will monitor more from 10-100 AU than 200,000 bright stars in the solar neighborhood at Eric Nielsen1; Robert De Rosa1; Bruce Macintosh1; a two minute cadence for drops in brightness caused Jason Wang2; Jean-Baptiste Ruffio1; Eugene Chiang3; by planetary transits. This first-ever spaceborne all- Mark Marley4; Didier Saumon5; Dmitry Savransky6; sky transit survey is identifying planets ranging in Daniel Fabrycky7; Quinn Konopacky8; Jennifer size from Earth-sized to gas giants, orbiting a wide Patience9; Vanessa Bailey10 variety of host stars, from cool M dwarfs to hot O/B 1 KIPAC, Stanford University (Stanford, California, United States) giants. 2 Jet Propulsion Laboratory, California Institute of Technology TESS stars are typically 30–100 times brighter than (Pasadena, California, United States) those surveyed by the Kepler satellite; thus, TESS 3 Astronomy, California Institute of Technology (Pasadena, Califor- planets are proving far easier to characterize with nia, United States) follow-up observations than those from prior mis- 4 Astronomy, U.C. -

Sized Exoplanet Thomas Barclay1,2, Jason F
A sub-Mercury-sized exoplanet Thomas Barclay1,2, Jason F. Rowe1,3, Jack J. Lissauer1, Daniel Huber1,4, François Fressin5, Steve B. Howell1, Stephen T. Bryson1, William J. Chaplin6, Jean-Michel Désert5, Eric D. Lopez7, Geoffrey W. Marcy8, Fergal Mullally1,3, Darin Ragozzine5,9, Guillermo Torres5, Elisabeth R. Adams5, Eric Agol10, David Barrado11,12, Sarbani Basu13, Timothy R. Bedding14, Lars A. Buchhave15,16, David Charbonneau5, Jessie L. Christiansen1,3, Jørgen Christensen-Dalsgaard17, David Ciardi18, William D. Cochran19, Andrea K. Dupree5, Yvonne Elsworth6, Mark Everett20, Debra A. Fischer13, Eric B. Ford9, Jonathan J. Fortney7, John C. Geary5, Michael R. Haas1, Rasmus Handberg17, Saskia Hekker6,21, Christopher E. Henze1, Elliott Horch22, Andrew W. Howard23, Roger C. Hunter1, Howard Isaacson8, Jon M. Jenkins1,3, Christoffer Karoff17, Steven D. Kawaler24, Hans Kjeldsen17, Todd C. Klaus25, David W. Latham5, Jie Li1,3, Jorge Lillo-Box12, Mikkel N. Lund17, Mia Lundkvist17, Travis S. Metcalfe26, Andrea Miglio6, Robert L. Morris1,3, Elisa V. Quintana1,3, Dennis Stello14, Jeffrey C. Smith1,3, Martin Still1,2, & Susan E. Thompson1,3 1 NASA Ames Research Center, Moffett Field, CA 94035, USA 2 Bay Area Environmental Research Institute, 596 First St West, Sonoma, CA 95476, USA 3 SETI Institute, 189 Bernardo Ave, Mountain View, CA 94043, USA 4 NASA Postdoctoral Program Fellow 5 Harvard-Smithsonian Center for Astrophysics, 60 Garden Street, Cambridge, MA 02138, USA 6 School of Physics and Astronomy, University of Birmingham, Edgbaston, B15 2TT, UK 7 Department of Astronomy and Astrophysics, University of California, Santa Cruz, CA 95064, USA 8 Department of Astronomy, UC Berkeley, Berkeley, CA 94720, USA 9 Astronomy Department, University of Florida, 211 Bryant Space Sciences Center, Gainesville, FL 32111, USA 10 Department of Astronomy, Box 351580, University of Washington, Seattle, WA 98195, USA 11 Calar Alto Observatory, Centro Astronómico Hispano Alemán, C/ Jesús Durbán Remón, E- 04004 Almería, Spain 12 Depto.