Ischemic Bowel Disease in 2021
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Twisted Bowels: Intestinal Obstruction Blake Briggs, MD Mechanical
Twisted Bowels: Intestinal obstruction Blake Briggs, MD Objectives: define bowel obstructions and their types, pathophysiology, causes, presenting signs/symptoms, diagnosis, and treatment options, as well as the complications associated with them. Bowel Obstruction: the prevention of the normal digestive process as well as intestinal motility. 2 overarching categories: Mechanical obstruction: More common. physical blockage of the GI tract. Can be complete or incomplete. Complete obstruction typically is more severe and more likely requires surgical intervention. Functional obstruction: diffuse loss of intestinal motility and digestion throughout the intestine (e.g. failure of peristalsis). 2 possible locations: Small bowel: more common Large bowel All bowel obstructions have the potential risk of progressing to complete obstruction Mechanical obstruction Pathophysiology Mechanical blockage of flow à dilation of bowel proximal to obstruction à distal bowel is flattened/compressed à Bacteria and swallowed air add to the proximal dilation à loss of intestinal absorptive capacity and progressive loss of fluid across intestinal wall à dehydration and increasing electrolyte abnormalities à emesis with excessive loss of Na, K, H, and Cl à further dilation leads to compression of blood supply à intestinal segment ischemia and resultant necrosis. Signs/Symptoms: The goal of the physical exam in this case is to rule out signs of peritonitis (e.g. ruptured bowel). Colicky abdominal pain Bloating and distention: distention is worse in distal bowel obstruction. Hyperresonance on percussion. Nausea and vomiting: N/V is worse in proximal obstruction. Excessive emesis leads to hyponatremic, hypochloremic metabolic alkalosis with hypokalemia. Dehydration from emesis and fluid shifts results in dry mucus membranes and oliguria Obstipation: severe constipation or complete lack of bowel movements. -

Isquemia Gástrica Secundaria a Estenosis Crítica Del Tronco Celíaco Doi.Org/10.23938/ASSN.0248
NOTAS CLÍNICAS0 Gastric ischemia due to critical stenosis of the celiac trunk Isquemia gástrica secundaria a estenosis crítica del tronco celíaco doi.org/10.23938/ASSN.0248 C. Saldaña Dueñas, A. Elosua González, A. Guerra Lacunza Contenido Gastric ischemia due to critical stenosis of the celiac trunk 123 ABSTRACT RESUMEN Abstract 123 Gastric ischemia (GI) results from diffuse or localized La isquemia gástrica resulta de la insuficiencia vascular Resumen 123 vascular insufficiency caused by different aetiologies difusa o localizada causada por diferentes etiologías such as systemic hypotension, vasculitis, disseminated como la hipotensión sistémica, la vasculitis, el trom- INTRODUCTION 124 thromboembolism and celiac or mesenteric stenosis. boembolismo diseminado y la estenosis mesentérica We present a case of gastric ischemia due to critical o celíaca. Presentamos un caso de isquemia gástrica CASE REPORT 124 stenosis of the celiac artery treated using endovascular secundaria a estenosis crítica del tronco celíaco trata- DISCUSSION 125 therapy. The celiac artery is the first major branch of da endovascularmente. El tronco celíaco es la primera the abdominal aorta and provides some of the blood rama de la aorta abdominal y aporta gran parte del flujo REFERENCES 127 supply to the stomach through the left gastric artery de sangre al estómago a través de la arteria gástrica iz- and other organs like the spleen (splenic artery branch) quierda y de otros órganos como el bazo (a través de la and the liver. Although the collateral blood supply to rama esplénica) y el hígado. Aunque las colaterales que the stomach is protective, systemic hypotension or irrigan el estómago son protectoras, la hipotensión sis- occlusion of the main arteries, as in the case of our pa- témica o la oclusión de las principales ramas como en tient, may result in gastric ischemia. -

Evaluation of Abdominal Pain in the Emergency Department Hartmut Gross, M.D., FACEP
Evaluation of Abdominal Pain in the Emergency Department Hartmut Gross, M.D., FACEP Abdominal pain complaints comprise about 5% of all Emergency Department visits. The etiology of the pain may be any of a large number of processes. Many of these causes will be benign and self-limited, while others are medical urgencies or even surgical emergencies. As with any complaint in the ED, the worst diagnosis is always entertained first. Therefore, there is one thought, which the ED practitioner must maintain in the foreground of his mind: “Is there a life threatening process?” Etiology A breakdown of the most common diagnoses of abdominal pain presentations is listed below. Note that nearly half of the time, “unknown origin” is the diagnosis made. This is a perfectly acceptable conclusion, after a proper work-up has ruled out any life threatening illness. Common Diagnoses of Non-traumatic Abdominal Pain in the ED 1 Abdominal pain of unknown origin 41.3% 2 Gastroenteritis 6.9% 3 Pelvic Inflammatory Disease 6.7% 4 Urinary Tract Infection 5.2% 5 Ureteral Stone 4.3% 6 Appendicitis 4.3% 7 Acute Cholecystitis 2.5% 8 Intestinal Obstruction 2.5% 9 Constipation 2.3% 10 Duodenal Ulcer 2.0% 11 Dysmenorrhea 1.8% 12 Simple Pregnancy 1.8% 13 Pyelonephritis 1.7% 14 Gastritis 1.4% 15 Other 12.8% From Brewer, RJ., et al, Am J Surg 131: 219, 1976. Two important factors modify the differential diagnosis in patients who present with abdominal pain: sex and age. Other common diagnoses of abdominal pain in men and women are as follows. -

Congenital Internal Hernia Through Defect in the Falciform Ligament in Adult
CASE REPORT – OPEN ACCESS International Journal of Surgery Case Reports 26 (2016) 104–107 Contents lists available at ScienceDirect International Journal of Surgery Case Reports j ournal homepage: www.casereports.com Congenital internal hernia through defect in the falciform ligament in adult: A case report and review of the literature ∗ Simona Macina , Tommaso Testa, Caterina Losacco University of study of Genoa (Italy) IRCCS, Largo Benzi 10, 16132 Genova, Italy a r t a b i c s t l r e i n f o a c t Article history: INTRODUCTION: The incidence of occlusion syndrome caused by internal hernia is very rare, in particular Received 22 March 2016 when the defect is congenital discovered in adults with no previous abdominal surgery. Accepted 1 May 2016 PRESENTATION OF CASE: We present a case of a 31 year-old female patient who presented with acute Available online 17 May 2016 abdominal pain and mechanical obstruction. The patient had never undergone abdominal surgery. DISCUSSION: On diagnostic laparoscopy, it was found a herniation of a loop of small bowel through a Keywords: hole in the falciform ligament. The obstruction was solved by the division of part of falciform ligament Internal hernia without intestinal resection. Falciform ligament CONCLUSION: Internal hernia is a very uncommon pathology, most often discovered in pediatric age because of congenital abnormalities, it must be included in the differential diagnosis in adults. Preoper- ative diagnosis is difficult. The diagnostic laparoscopic approach has shown to be the best. © 2016 Published by Elsevier Ltd on behalf of IJS Publishing Group Ltd. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/). -

Gastroparesis and Dumping Syndrome: Current Concepts and Management
Journal of Clinical Medicine Review Gastroparesis and Dumping Syndrome: Current Concepts and Management Stephan R. Vavricka 1,2,* and Thomas Greuter 2 1 Center of Gastroenterology and Hepatology, CH-8048 Zurich, Switzerland 2 Department of Gastroenterology and Hepatology, University Hospital Zurich, CH-8091 Zurich, Switzerland * Correspondence: [email protected] Received: 21 June 2019; Accepted: 23 July 2019; Published: 29 July 2019 Abstract: Gastroparesis and dumping syndrome both evolve from a disturbed gastric emptying mechanism. Although gastroparesis results from delayed gastric emptying and dumping syndrome from accelerated emptying of the stomach, the two entities share several similarities among which are an underestimated prevalence, considerable impairment of quality of life, the need for a multidisciplinary team setting, and a step-up treatment approach. In the following review, we will present an overview of the most important clinical aspects of gastroparesis and dumping syndrome including epidemiology, pathophysiology, presentation, and diagnostics. Finally, we highlight promising therapeutic options that might be available in the future. Keywords: gastroparesis; dumping syndrome; pathophysiology; clinical presentation; treatment 1. Introduction Gastroparesis and dumping syndrome both evolve from a disturbed gastric emptying mechanism. While gastroparesis results from significantly delayed gastric emptying, dumping syndrome is a consequence of increased flux of food into the small bowel [1,2]. The two entities share several important similarities: (i) gastroparesis and dumping syndrome are frequent, but also frequently overlooked; (ii) they affect patient’s quality of life considerably due to possibly debilitating symptoms; (iii) patients should be taken care of within a multidisciplinary team setting; and (iv) treatment should follow a step-up approach from dietary modifications and patient education to pharmacological interventions and, finally, surgical procedures and/or enteral feeding. -

Jejunoileal Diverticulitis: Big Trouble in Small Bowel
Jejunoileal Diverticulitis: Big Trouble in Small Bowel MICHAEL CARUSO DO, HAO LO MD EMERGENCY RADIOLOGY, UMASS MEMORIAL MEDICAL CENTER, WORCESTER, MA LEARNING OBJECTIVES: After excluding Meckel’s diverticulum, less than 30% of reported diverticula occurred in the CASE 1 1. Be aware of the relatively rare diagnoses of jejunoileal jejunum and ileum. HISTORY: 52 year old female with left upper quadrant pain. diverticulosis (non-Meckelian) and diverticulitis. Duodenal diverticula are approximately five times more common than jejunoileal diverticula. FINDINGS: 2.2 cm diverticulum extending from the distal ileum with adjacent induration of the mesenteric fat, consistent with an ileal diverticulitis. 2. Learn the epidemiology, pathophysiology, imaging findings and differential diagnosis of jejunoileal diverticulitis. Diverticulaare sac-like protrusions of the bowel wall, which may be composed of mucosa and submucosa only (pseudodiverticula) or of all CT Findings (2) layers of the bowel wall (true . Usually presents as a focal area of bowel wall thickening most prominent on the mesenteric side of AXIAL AXIAL CORONAL diverticula) the bowel with adjacent inflammation and/or abscess formation . Diverticulitis is the result of When abscess is present, CT findings may include relatively smooth margins, areas of low CASE 2 obstruction of the neck of the attenuation within the mass, rim enhancement after IV contrast administration, gas within the HISTORY: 62 year old female with epigastric and left upper quadrant pain. diverticulum, with subsequent mass, displacement of the surrounding structures, and edema of thickening of the surrounding fat inflammation, perforation and or fascial planes FINDINGS: 4.7 cm diameter out pouching seen arising from the proximal small bowel and associated with adjacent inflammatory stranding. -

Ischaemic Colitis: Practical Challenges and Evidence-Based Recommendations For
Ischaemic Colitis: Practical Challenges and Evidence-Based Recommendations for Management Alexander Hung1*, Tom Calderbank1*, Mark A Samaan1, Andrew A Plumb2, George Webster1 Author affiliations 1Department of Gastroenterology, University College London Hospitals, London, UK 2Department of Radiology, University College London Hospitals, London, UK *Authors contributed equally Correspondence to Mark A Samaan Department of Gastroenterology University College London Hospitals 250 Euston Road London NW1 2PG [email protected] Running title Ischaemic Colitis Management Word count 3212 Key Words Ischaemic colitis, colonic ischaemia ABSTRACT Ischaemic colitis (IC) is a common condition with rising incidence and, in severe cases, a high mortality rate. Its presentation, severity and disease behaviour can vary widely and there exists significant heterogeneity in treatment strategies and resultant outcomes. In this article we explore practical challenges in the management of IC and where available, make evidence-based recommendations for its management based on a comprehensive review of available literature. An optimal approach to initial management requires early recognition of the diagnosis followed by prompt and appropriate investigation. Ideally, this should involve the input of both gastroenterology and surgery. CT with intravenous and oral contrast is the imaging modality of choice. It can support a clinical diagnosis, define the severity and distribution of ischaemia and has prognostic value. In all but fulminant cases, this should be followed (within 48 hours) by lower GI endoscopy to reach the distal-most extent of the disease, providing endoscopic (and histological) confirmation. The mainstay of medical management is conservative/supportive treatment, with bowel rest, fluid resuscitation and antibiotics. Specific laboratory, radiological and endoscopic features are recognised to correlate with more severe disease, higher rates of surgical intervention and ultimately worse outcomes. -

Coping with the Problems of Diagnosis of Acute Colitis
Diagnostic and Interventional Imaging (2013) 94, 793—804 . CONTINUING EDUCATION PROGRAM: FOCUS . Coping with the problems of diagnosis of acute colitis a,∗,c b a E. Delabrousse , F. Ferreira , N. Badet , a b M. Martin , M. Zins a Urinogenital and Digestive Imaging Department, hôpital Jean-Minjoz, CHRU de Besanc¸on, 3, boulevard Fleming, 25030 Besanc¸on, France b Radiology Department, hôpital Saint-Joseph, 184, rue Raymond-Losserand, 75014 Paris, France c EA 4662, Nanomedicine Laboratory, Imagery and Therapeutics, University of Franche-Comté, Besanc¸on, France KEYWORDS Abstract Acute colitis is an acute condition of the colon. For the radiologist, it is mainly Colitis; diagnosed during differential diagnosis of acute abdominal conditions. There are many causes Ischaemia; of colitis and the degree of its severity varies. A CT scan is the best imaging examination for IBD; diagnosing it and also for analysing and characterising colitis. The topography, type of lesion Pseudomembranous; and associated factors can often suggest a precise diagnosis but it is nevertheless essential to Neutropenia integrate these findings into the clinical context and take laboratory values into account. The use of endoscopy is still the rule where a doubt remains, or to obtain necessary histological evidence. © 2013 Éditions françaises de radiologie. Published by Elsevier Masson SAS. All rights reserved. Acute colitis is an acute condition of the colon and most often presents in the form of an acute abdominal picture with very variable clinical symptoms and laboratory test results. The most frequent symptoms encountered in colitis are abdominal pain, fever and diarrhoea [1]. Hyperleucocytosis and elevated CRP in laboratory tests are common. -

Ischemic Colitis Caused by Polycythemia Vera: a Case Report and Literature Review
EXPERIMENTAL AND THERAPEUTIC MEDICINE 16: 3663-3667, 2018 Ischemic colitis caused by polycythemia vera: A case report and literature review SHASHA ZHANG1, RUIXUE LAI1, XUEQING GAO1, YUFEI ZHAO1, YUE ZHAO2, JIANHUA WU3 and ZHANJUN GUO1 Departments of 1Rheumatology and Immunology, and 2Gastroenterology and Hepatology, 3Animal Center, The Fourth Hospital of Hebei Medical University, Shijiazhuang, Hebei 050011, P.R. China Received January 16, 2018; Accepted August 10, 2018 DOI: 10.3892/etm.2018.6638 Abstract. Polycythemia vera (PV) is a chronic myeloproliferative Ischemic colitis (IC) can be described as an ischemic disease disorder originating from hematopoietic stem cells and of the colon, which results from decreased blood flow due to complicated by thrombosis and bleeding. This report describes a various causes that leads to intestinal wall ischemia, injury or case of ischemic colitis (IC) caused by PV and includes a review necrosis (3). The risk factors for IC mainly include diabetes, of the relevant literature. The patient was a 59-year-old male hypertension, dyslipidemia, peripheral vascular disease, with a history of PV who presented with abdominal pain and aspirin administration, and digoxin or constipation-inducing hematochezia. Colonoscopy and histopathological examination medications (4). The main clinical manifestations include results indicated suspected ischemic bowel disease. Following abdominal pain, diarrhea, and hematochezia (5). A reliable experimental anticoagulant therapy for 7 days, the patient no diagnosis of IC depends on the comprehensive evaluation of longer experienced abdominal pain and hematochezia had clinical symptoms, biochemical tests, radiological examina- resolved. Colonoscopy review showed no obvious anomalies tions, and endoscopic assessments (6). In certain cases, PV 1 month later. -
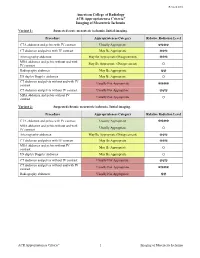
ACR Appropriateness Criteria® Imaging of Mesenteric Ischemia
Revised 2018 American College of Radiology ACR Appropriateness Criteria® Imaging of Mesenteric Ischemia Variant 1: Suspected acute mesenteric ischemia. Initial imaging. Procedure Appropriateness Category Relative Radiation Level CTA abdomen and pelvis with IV contrast Usually Appropriate ☢☢☢☢ CT abdomen and pelvis with IV contrast May Be Appropriate ☢☢☢ Arteriography abdomen May Be Appropriate (Disagreement) ☢☢☢ MRA abdomen and pelvis without and with May Be Appropriate (Disagreement) IV contrast O Radiography abdomen May Be Appropriate ☢☢ US duplex Doppler abdomen May Be Appropriate O CT abdomen and pelvis without and with IV Usually Not Appropriate contrast ☢☢☢☢ CT abdomen and pelvis without IV contrast Usually Not Appropriate ☢☢☢ MRA abdomen and pelvis without IV Usually Not Appropriate contrast O Variant 2: Suspected chronic mesenteric ischemia. Initial imaging. Procedure Appropriateness Category Relative Radiation Level CTA abdomen and pelvis with IV contrast Usually Appropriate ☢☢☢☢ MRA abdomen and pelvis without and with Usually Appropriate IV contrast O Arteriography abdomen May Be Appropriate (Disagreement) ☢☢☢ CT abdomen and pelvis with IV contrast May Be Appropriate ☢☢☢ MRA abdomen and pelvis without IV May Be Appropriate contrast O US duplex Doppler abdomen May Be Appropriate O CT abdomen and pelvis without IV contrast Usually Not Appropriate ☢☢☢ CT abdomen and pelvis without and with IV Usually Not Appropriate contrast ☢☢☢☢ Radiography abdomen Usually Not Appropriate ☢☢ ACR Appropriateness Criteria® 1 Imaging of Mesenteric Ischemia IMAGING OF MESENTERIC ISCHEMIA Expert Panels on Vascular Imaging and Gastrointestinal Imaging: Michael Ginsburg, MDa; Piotr Obara, MDb; Drew L. Lambert, MDc; Michael Hanley, MDd; Michael L. Steigner, MDe; Marc A. Camacho, MD, MSf; Ankur Chandra, MDg; Kevin J. Chang, MDh; Kenneth L. -

Abdominal Pain
Abdominal Pain 1 Given a patient with abdominal pain, paying particular attention to its location and chronicity: a) Distinguish between acute and chronic pain. Arbitrary number cannot be used to distinguish between acute and chronic pain. Pain of less than a few days duration that has worsened progressively until the time of presentation may be classified as acute. Pain that has remained unchanged for months or years may be classified as chronic. Pain that does not clearly fit either category might be called subacute and requires consideration of the differential diagnoses for both acute and chronic pain. b) Generate a complete differential diagnosis (ddx). GI tract Inflammatory: gastroenteritis, appendicitis, gastritis, esophagitis, diverticulitis, Crohn's disease, ulcerative colitis, microscopic colitis Obstruction: hernia, intussusception, volvulus, post-surgical adhesions, tumours, superior mesenteric artery syndrome, severe constipation, hemorrhoids Vascular: embolism, thrombosis, hemorrhage, sickle cell disease, abdominal angina, blood vessel compression (such as celiac artery compression syndrome), Digestive: peptic ulcer, lactose intolerance, coeliac disease, food allergies Bile system Inflammatory: cholecystitis, cholangitis Obstruction: cholelithiasis, tumours Liver Inflammatory: hepatitis, liver abscess Pancreatic Inflammatory: pancreatitis Renal and urological Inflammation: pyelonephritis, bladder infection Obstruction: kidney stones, urolithiasis, Urinary retention, tumours Vascular: left renal vein entrapment Gynaecological -

Small Bowel Infarction by Aspergillus
short report Haematologica 1997; 82:182-183 SMALL BOWEL INFARCTION BY ASPERGILLUS LUCIO CATALANO, MARCO PICARDI, DARIO ANZIVINO,* LUIGI INSABATO,° ROSARIO NOTARO, BRUNO ROTOLI Cattedre di Ematologia, *Microbiologia e °Anatomia Patologica, Università Federico II, Naples, Italy ABSTRACT Primary gut involvement by Aspergillus is a rare for small bowel perforation. The patient died a and often fatal complication of intensive anti- week later in spite of amphotericin-B treatment leukemic therapy. We describe the case of an adult and neutrophil recovery. Anti-Aspergillus prophy- patient affected by acute leukemia who developed laxis and early introduction of amphotericin-B in a small bowel fungal thromboembolism without the treatment of febrile neutropenia is probably radiographic evidence of lung involvement during advisable in all cases of AML. the post-induction aplastic phase. The diagnosis ©1997, Ferrata Storti Foundation was made histologically at laparotomy performed Key words: Aspergillosis, small bowel, mycotic thromboembolism linical reports of gut involvement by revealed diffuse mycotic infiltration of the intestinal Aspergillus with arterial infarctions are rare.1-3 wall with fungal emboli obturating the lumen of CWe report on an adult male affected by a the superior mesenteric artery branches (Figures 1 favorable subtype of acute myeloid leukemia (AML and 2). Colonies of A. fumigatus grew from bowel M4-Eo) who succumbed to fatal intestinal asper- fragment cultures. Melena persisted despite neu- gillosis (IA) after effective induction treatment. trophil recovery (which was accelerated by adminis- tration of G-CSF at 300 µg/d for 7 days) and treat- Case report ment with 50 mg/d liposomal amphotericin, cho- AML, FAB M4-Eo with cytogenetic evidence of sen because the patient was in severe renal failure inv 16, was diagnosed in a 58-year-old male in (plasma creatinine 5 mg/dL).