Trifluoroacetic Acid Recovery from Industrial Aqueous Effluent
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

1,1,1,2-Tetrafluoroethane
This report contains the collective views of an international group of experts and does not necessarily represent the decisions or the stated policy of the United Nations Environment Programme, the International Labour Organisation, or the World Health Organization. Concise International Chemical Assessment Document 11 1,1,1,2-Tetrafluoroethane First draft prepared by Mrs P. Barker and Mr R. Cary, Health and Safety Executive, Liverpool, United Kingdom, and Dr S. Dobson, Institute of Terrestrial Ecology, Huntingdon, United Kingdom Please not that the layout and pagination of this pdf file are not identical to the printed CICAD Published under the joint sponsorship of the United Nations Environment Programme, the International Labour Organisation, and the World Health Organization, and produced within the framework of the Inter-Organization Programme for the Sound Management of Chemicals. World Health Organization Geneva, 1998 The International Programme on Chemical Safety (IPCS), established in 1980, is a joint venture of the United Nations Environment Programme (UNEP), the International Labour Organisation (ILO), and the World Health Organization (WHO). The overall objectives of the IPCS are to establish the scientific basis for assessment of the risk to human health and the environment from exposure to chemicals, through international peer review processes, as a prerequisite for the promotion of chemical safety, and to provide technical assistance in strengthening national capacities for the sound management of chemicals. The Inter-Organization -

Functionalization of Heterocycles: a Metal Catalyzed Approach Via
FUNCTIONALIZATION OF HETEROCYCLES: A METAL CATALYZED APPROACH VIA ALLYLATION AND C-H ACTIVATION A Dissertation Submitted to the Graduate Faculty of the North Dakota State University of Agriculture and Applied Science By Sandeepreddy Vemula In Partial Fulfillment of the Requirements for the Degree of DOCTOR OF PHILOSOPHY Major Department: Chemistry and Biochemistry October 2018 Fargo, North Dakota North Dakota State University Graduate School Title FUNCTIONALIZATION OF HETEROCYCLES: A METAL CATALYZED APPROACH VIA ALLYLATION AND C-H ACTIVATION By Sandeepreddy Vemula The Supervisory Committee certifies that this disquisition complies with North Dakota State University’s regulations and meets the accepted standards for the degree of DOCTOR OF PHILOSOPHY SUPERVISORY COMMITTEE: Prof. Gregory R. Cook Chair Prof. Mukund P. Sibi Prof. Pinjing Zhao Prof. Dean Webster Approved: November 16, 2018 Prof. Gregory R. Cook Date Department Chair ABSTRACT The central core of many biologically active natural products and pharmaceuticals contain N-heterocycles, the installation of simple/complex functional groups using C-H/N-H functionalization methodologies has the potential to dramatically increase the efficiency of synthesis with respect to resources, time and overall steps to key intermediate/products. Transition metal-catalyzed functionalization of N-heterocycles proved as a powerful tool for the construction of C-C and C-heteroatom bonds. The work in this dissertation describes the development of palladium catalyzed allylation, and the transition metal catalyzed C-H activation for selective functionalization of electron deficient N-heterocycles. Chapter 1 A thorough study highlighting the important developments made in transition metal catalyzed approaches for C-C and C-X bond forming reactions is discussed with a focus on allylation, directed indole C-2 substitution and vinylic C-H activation. -

Ion Chromatographic Analysis of Disinfectant By-Products
DCU Ion Chromatographic Analysis of Disinfectant By-Products For the award of D octor of Philosophy Leon Barron School of Chem ical Sciences Student ID: 97311006 V S > N C S R National Centra for Sansor Raiaireh Table of Contents Declaration 11 List of Figures 12 List of Tables 20 List of Abbreviations 22 List of Publications 23 List of Poster Presentations 24 List of Oral Presentations 25 List of Honours and A wards 2 5 Acknowledgements 26 Abstract 28 Chapter 1.0 Literature Review 30 1.0 Background 31 1.1 Current Water Treatment Methods 32 1.1.1 Ozonation 32 1.1.2 Chlorination 35 1.2 Factors Affecting the Formation of Disinfectant By- 36 Products 1.2.1 pH 36 1.2.2 Contact Time 37 1.2.3 Temperature and Season 37 1.2.4 Concentration and Properties of Natural Organic Matter 37 1.2.5 Concentration of Chlorine and Residual Chlorine 38 1.2.6 Concentration of Bromide 38 2 1.3 The Haloacetic Acids 39 1.3.1 Chlorinated Acetic Acids 39 1.3.1.1 Physical and Chemical Properties 39 1.3.1.2 Effects on Laboratory Animals and/or 39 Humans 1.3.2 Brominated Acetic Acids 40 1.3.2.1 Physical Properties 40 1.3.2.2 Effect on Laboratory Animals and/or 41 Humans 1.4 Chromatographic Determination of Haloacetic Acids 42 1.4.1 Introduction to chromatographic theory 42 1.4.2 Band broadening in chromatography 45 1.4.2.1 Multiple path lengths 45 1.4.2.3 Molecular diffusion 46 1.4.2.4 Mass transfer 47 1.4.2.5 The van Deemter equation 47 1.5 Ion Exchange Chromatography 48 1.5.1 The Ion Exchange Process 49 1.5.1.1 Cation Exchange 49 1.5.1.2 Anion Exchange 49 1.5.2 Ion -

Prepared by 3M
DRAFT ECOTOXICOLOGY AND ENVIRONMENTAL FATE TESTING OF SHORT CHAIN PERFLUOROALKYL COMPOUNDS RELATED TO 3M CHEMISTRIES XXX XX, 2008 Prepared by 3M Page 1 CONFIDENTIAL - SUBJECT TO A PROTECTIVE ORDER ENTERED IN 3M MN01537089 HENNEPIN COUNTY DISTRICT COURT, NO. 27-CV-10-28862 2231.0001 DRAFT TABLE OF CONTENTS Page Introduction (describes propose, goals, document organization .... ) 3 1) Degradation modes 4-27 Basic degradation pathways, Conversion routes from polymer to monomer & degradants or residual intermediates to degradants) 2) Degradants & Intermediates - Phys. Chem properties and test data by section & chapter i) Fluorinated Sulfonic acids and derivatives a) PFBS (PBSK) 28-42 b) PFBSI 43-44 ii) Fluorinated Carboxylates a) TFA 46-55 b) PFPA 56-59 c) PFBA (APFB) 60-65 d) MeFBSAA (MeFBSE Acid, M370) 66-68 iii) Fluorinated Non-ionics a) MeFBSE 70-74 b) MeFBSA 75-80 c) FBSE 81-84 d) FBSA 85-88 e) HxFBSA 89-91 iv) Fluorinated Inerts & volatiles a) PBSF 94-97 b) NFB, C4 hydride, CF3CF2CF2CF2-H 98-99 3) Assessments X Glossary 100-102 References/Endnotes 105- Page 2 CONFIDENTIAL - SUBJECT TO A PROTECTIVE ORDER ENTERED IN 3M MN01537090 HENNEPIN COUNTY DISTRICT COURT, NO. 27-CV-10-28862 2231.0002 DRAFT Introduction to Environmental White Papers on Perfluoroalkyl acids related to 3M chemistries Perfluorochemicals have been commonplace in chemical industry over 50 years but until recently there has been little information on environmental fate and effects avialble in open literature. The following chapters summarize the findings of"list specific C4 intermediates PFBS, PFBSI, PFBA, PFPA, MeFBSAA, TFA, MeFBSE, FBSA, HxFBSA, FBSE, PBSF, NFB " As background, 3M announced on May 16, 2000 the voluntary manufacturing phase out of perfluorooctanyl chemicals which included perfluorooctanoic acid (PFOA), perfluorooctane sulfonate (PFOS), and PFOS-related chemistries. -

The Background Ion Master List Includes
REV. A BACKGROUND ION MASTER LIST 1 OF 5 The background ion master list includes: • ESI+ common background ions on page 1 • ESI- common background ions on page 4 • ESI+ common clusters on page 4 • ESI+ common adducts on page 5 • ESI+ ion series on page 5 Table 1: ESI+ common background ions m/z Ion Compound Source 33 (M+H)+ methanol 42 (M+H)+ acetonitrile + 59 (M+NH4) acetonitrile 64 (M+Na)+ acetonitrile 65 (2M+H)+ methanol 74 (M+H)+ dimethylformamide 79 (M+H)+ DMSO 83 (2M+H)+ acetonitrile 85 (M+H)+ d6-DMSO 88 (M+formic acid+H)+ acetonitrile 101 (M+Na)+ DMSO 102 (M+H)+ triethylamine 104/106 (M+Cu)+ acetonitrile 105 (2M+Na)+ acetonitrile 115 (M+dimethyl formamide+H)+ acetonitrile + 120 (M+Na+CH3CN) DMSO 122 (M+H)+ tris (tris(hydroxymethyl)aminomethane) 123 (M+H)+ diisopropylethylamine 130 (M+H)+ diisopropylethylamine + 137 (M+CH3CN+NH4) DMSO 144 (M+H)+ tripropylamine 145/147 (2M+Cu)+ acetonitrile 146 (3M+Na)+ acetonitrile 149 (M+H)+ phthalic anhydride 150 (M+H)+ phenyldiethylamine 153 (M+H)+ 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) 157 (2M+H)+ DMSO 159 (M+Na)+ sodium trifluoroacetate 163 dimethyl phthalate 167 dioctyl phthalate 169 (2M+H)+ d6-DMSO 171 (M+Na)+ phthalic anhydride 179 (2M+Na)+ DMSO 186 (M+H)+ tributylamine 195 (M+H)+ dimethyl phthalate 214 (M+H)+ n-butylbenzenesulfonamide plasticizer © 2010 WATERS COPORATION. ALL RIGHTS RESERVED. REV. A BACKGROUND ION MASTER LIST 2 OF 5 Table 1: ESI+ common background ions (continued) m/z Ion Compound Source 225 (M+H)+ dicyclohexylurea + 231 (M+NH4) n-butyl benzenesulfonamide plasticizer -

Poly(Limonene Carbonate): a Bio-Based & Versatile High-Performance Thermoplastic
Poly(limonene carbonate): a bio-based & versatile high-performance thermoplastic Dissertation zur Erlangung des akademischen Grades eines Doktors der Naturwissenschaften (Dr. rer. Nat.) an der Bayreuther Graduiertenschule für Mathematik und Naturwissenschaften (BayNAT) der Universität Bayreuth vorgelegt von Oliver Hauenstein aus Dortmund Bayreuth 2016 Die vorliegende Arbeit wurde in der Zeit von November 2012 bis Juni 2016 in Bayreuth am Lehrstuhl Makromolekulare Chemie II unter Betreuung von Herrn Professor Dr. Andreas Greiner angefertigt. Vollständiger Abdruck der von der Bayreuther Graduiertenschule für Mathematik und Naturwissenschaften (BayNAT) der Universität Bayreuth genehmigten Dissertation zur Erlangung des akademischen Grades eines Doktors der Naturwissenschaften (Dr. rer. nat.). Dissertation eingereicht am: 13.07.2016 Zulassung durch das Leitungsgremium: 26.07.2016 Wissenschaftliches Kolloquium: 01.02.2017 Amtierender Direktor: Prof. Dr. Stephan Kümmel Prüfungsausschuss: Prof. Dr. Andreas Greiner (Erstgutachter) Prof. Dr. Hans-Werner Schmidt (Zweitgutachter) Prof. Dr. Carlo Unverzagt. (Vorsitz) Prof. Dr. Jürgen Senker Drittgutachter: Prof. Dr. Volker Abetz Für meine Familie & Hui Contents Abbreviations & symbols ......................................................................................................................... i List of publications ................................................................................................................................... v Abstract .................................................................................................................................................... -

United States Patent (19) 11 Patent Number: 4,626,325 Trummlitz Et Al
United States Patent (19) 11 Patent Number: 4,626,325 Trummlitz et al. (45) Date of Patent: Dec. 2, 1986 54 PROCESS FOR THE PREPARATION OF (56) References Cited 4-HYDROXY-1,2-BENZISOTHIAZOL U.S. PATENT DOCUMENTS 3(2H)-ONE-1,1-DIOXIDE 4,057,555 11/1977 Kolke et al. ........................ 548/210 75 Inventors: Gunter Trummlitz, Warthausen; 4,140,697 2/1979 Batcho et al. ....................... 426/548 Wolfgang Eberlein, Biberach; 4,404,230 9/1983 Trummlitz et al. ................. 426/548 Wolfhard Engel, Biberach; Gerhard Primary Examiner-Arthur P. Demers Mihm, Biberach, all of Fed. Rep. of Attorney, Agent, or Firm-David E. Frankhouser; Alan Germany R. Stempel 73) Assignee: Karl Thomae GmbH, Biberach an der 57 ABSTRACT Riss, Fed. Rep. of Germany The specification describes a process for preparing 4 hydroxy-1,2-benzisothiazol-3(2H)-one-1,1-dioxide, a 21 Appl. No.: 841,392 sweetener, from 1,2-benzisothiazol-3(2H)-one-1,1-diox ide by anodic oxidation in the presence of trifluoroace (22 Filed: Mar. 19, 1986 tic acid or trifluoromethanesulfonic acid and, option 30 Foreign Application Priority Data ally, in the presence of salts which increase the conduc tivity. The oxidation is effected in an anhydrous me Mar. 19, 1985 DE Fed. Rep. of Germany ....... 35098.19 dium and the 4-trifluoroacetoxy-1,2-benzisothiazol 51) Int. Cl." ................................................ C25C3/00 3(2H)-one-1,1-dioxide formed as an intermediate when, 52 U.S. C. .................................. 204/59 R; 426/548; for example, trifluoroacetic acid is used is decomposed 548/210; 548/211 with water to obtain the desired end product. -
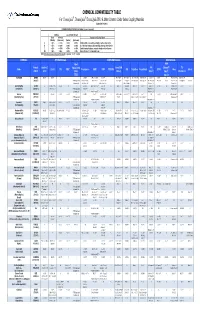
CPC Chemical Compatibility Chart
CHEMICAL COMPATIBILITY TABLE For ChemQuik®, DrumQuik®, DrumQuik PRO & Other Common Colder Series Coupling Materials (Updated 01/14/2010) INTERPRETATION OF TEST DATA (In 30 days to 1 year of exposure) Swelling Loss of Tensile Strength Linear Volumetric Description of Chemical Attack (Plastics) (Elastomers) (Plastics) (Elastomers) A < 10% <= 15% < 15% <=15% Excellent, little or no swelling, softening or surface deterioration B < 15% <= 30% < 30% <= 30% Good chemical resistance, minor swelling, softening or deterioration C < 20% <= 50% < 50% <= 60% Limited chemical resistance, moderate attack, conditional service NR > 20% > 50% > 50% > 60% Severe attack, not recommended for use NOTE: All temperatures are in degrees Fahrenheit. Conversion: °C = (°F - 32)/1.8 CHEMICAL SPRING Materials COUPLING Materials SEAL Materials Teflon® FFKM ® Formula Hastelloy C Encapsulated Acetal/POM FKM (Chemraz / TPO Name 316 SS PPS PEEK™ Polypropylene HDPE PVDF PTFE/PFA ABS Polysulfone Polycarbonate EPDM Buna Silicone (CAS #) (276) 316SS (Celcon) (Viton®) Simriz® / (Santoprene) (TESS) Kalrez®) Acetic Acid C2H4O2 A to 212° 212° A to 212° 212° A A A A to 140° 140° AB to 100% to 70° 70° A to 122° 122° A AAto5%to70 to 5% to 70°° AB 10% to 70° 70° A to 100% to 70° 70° A to 50% to 70° 70° A 10% to 70° 70° AAto70 to 70°° A B to 30% at 70° 70° A to 30% to 70° 70° A (64-19-7) (PTFE Encapsulated AB 50-100% to 160° AB 60% to 180° A to 10% to 225° BC 10% @ 70° C 20% @ 70° A to 20% to 140° B to 50% @ 122° B 10-25% to 100° AB to 200° A to 70° B to 20% to 185° C 50% @ 70° A to -

1,1,1,2-Tetrafluoroethane (HFC-134A) (CAS No. 811-97-2) (Second Edition)
1,1,1,2-Tetrafluoroethane (HFC-134a) (CAS No. 811-97-2) (Second Edition) JACC No. 50 ISSN-0773-6339-50 Brussels, January 2006 1,1,1,2-Tetrafluoroethane (HFC-134a) (CAS No. 811-97-2) (Second Edition) ECETOC JACC REPORT No. 50 © Copyright – ECETOC AISBL European Centre for Ecotoxicology and Toxicology of Chemicals 4 Avenue E. Van Nieuwenhuyse (Bte 6), B-1160 Brussels, Belgium. All rights reserved. No part of this publication may be reproduced, copied, stored in a retrieval system or transmitted in any form or by any means, electronic, mechanical, photocopying, recording or otherwise without the prior written permission of the copyright holder. Applications to reproduce, store, copy or translate should be made to the Secretary General. ECETOC welcomes such applications. Reference to the document, its title and summary may be copied or abstracted in data retrieval systems without subsequent reference. The content of this document has been prepared and reviewed by experts on behalf of ECETOC with all possible care and from the available scientific information. It is provided for information only. ECETOC cannot accept any responsibility or liability and does not provide a warranty for any use or interpretation of the material contained in the publication. ECETOC JACC No. 50 1,1,1,2-Tetrafluoroethane (HFC-134a) (CAS No. 811-97-2) (Second Edition) 1,1,1,2-Tetrafluoroethane (HFC-134a) (CAS No. 811-97-2) CONTENTS EXECUTIVE SUMMARY 1 THE ECETOC SCHEME FOR THE JOINT ASSESSMENT OF COMMODITY CHEMICALS 3 1. SUMMARY AND CONCLUSIONS 4 2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS 6 2.1 Identity 6 2.2 EU classification and labelling 6 2.3 Physical and chemical properties 6 2.4 Conversion factors 8 2.5 Analytical methods 8 3. -

With Halothane-Induced Hepatitis Are Directed Against a Trifluoroacetylated Carboxylesterase (Drug Hypersensitivity/Neoantigens/Metabolism) H
Proc. Natl. Acad. Sci. USA Vol. 86, pp. 322-326, January 1989 Medical Sciences Human anti-endoplasmic reticulum antibodies in sera of patients with halothane-induced hepatitis are directed against a trifluoroacetylated carboxylesterase (drug hypersensitivity/neoantigens/metabolism) H. SATOH*, B. M. MARTINt, A. H. SCHULICK*, D. D. CHRIST*, J. G. KENNA*, AND L. R. POHL*t *Laboratory of Chemical Pharmacology, National Heart, Lung and Blood Institute, and tClinical Neuroscience Branch, National Institute of Mental Health, National Institutes of Health, Bethesda, MD 20892 Communicated by Allan H. Conney, September 28, 1988 (receivedfor review July 5, 1988) ABSTRACT Previous studies have demonstrated that pa- covalently modified by the reactive TFA halide metabolite of tients with halothane-induced hepatitis have serum antibodies halothane (11). that are directed against novel liver microsomal neoantigens To investigate the role of the halothane-induced neoanti- and have suggested that these neoantigens may play an immu- gens in the pathogenesis of halothane hepatitis, a general nopathological role in development of the patients' liver dam- approach for their purification and characterization has been age. These investigations have further revealed that the anti- developed and utilized to identify one of them. bodies are directed against distinct polypeptide fractions (100 kDa, 76 kDa, 59 kDa, 57 kDa, 54 kDa) that have been covalently modified by the reactive trifluoroacetyl halide me- MATERIALS AND METHODS tabolite of halothane. In this paper, the trifluoroacetylated Purification of 59-kDa-TFA Protein from Halothane- (TFA) 59-kDa neoantigen (59-kDa-TFA) recognized by the Treated Rats by Immunoaffmnity and Anion-Exchange HPLC. patients' antibodies was isolated from liver microsomes of Specific anti-TFA IgG (12) was purified from antisera derived halothane-treated rats by chromatography on an immunoaf- from rabbits immunized with TFA rabbit serum albumin (13) finity column of anti-TFA IgG. -

Xii. Biological and Health Effects
XII. BIOLOGICAL AND HEALTH EFFECTS Combined Summary and Conclusions Toxicology of Atmospheric Degradation Products of Selected Hydrochlorofluorocarbons Laurence S. Kaminsky Department of Environmental Health & Toxicology School of Public Health State University of New York at Albany Empire State Plaza Albany, NY Assessment of Effects on Vegetation of Degradation Products from Alternative Fluorocarbons D. C. McCune and L. H. Weinstein Boyce Thompson Institute for Plant Research Ithaca, NY PRE,CEDJNG PA_E ,.,.,-,n_'_k,,.o_ NO'r. F?Lf_qED BIOLOGICALANDHEALTHEFFECTS COMBINED SUMMARY AND CONCLUSIONS There is a need for more information on exposure, especially to organic breakdown products. This should include estimates of ground level concentrations, modes of deposition (wet & dry) and should extend to identification of half-lives in soil and water, and the products of microbial transformation. Nothing is known of the toxicology to humans, animals or plants of any of the organic breakdown products other than trifluoroacetic acid. It was considered dubious to extrapolate from analogous compounds (e.g. trichloroacetic acid). The limited work on toxicology of TFA was with very high concentrations com- pared with those potentially arising from HCFC's and CFC's. Studies should aim to determine the long- term threshold level for toxicological effects. One of the major uncertainties is the fate of -CF3 as there is conflict of opinion about the stability of the C-F bond. The biological evidence (from toxicology and pesticide biochemistry) indicates that- CF3 is recalcitrant and may persist in the environment but an opinion was expressed that there may be significant chemical defluorination at room temperature. Because of the mammalian toxicity of monofluoracetate, the possibility of defluorination of trifluoro- to monofluoro- needs to be firmly clarified. -

Maria Atanassova Petrova the Crucial Performance of Mutual
Maria Atanassova Petrova The crucial performance of mutual solubility among water and ionic liquids in the time of liquid-liquid extraction of metallic species Academic Publications, Sofia 2020 The crucial performance of mutual solubility among water and ionic liquids in the time of liquid-liquid extraction of metallic species 2020, English language, First Edition Author: Dr. Eng. Maria Atanassova Petrova Scientific Reviewers: Assoc. Prof. Eng. Alexander Lirkov, PhD Assoc. Prof. Eng. Alexander Zahariev, PhD ISBN: 978-954-2940-23-4 Academic Publications Sofia, 2020 The photo on cover page was taken in the Aqua World Oarai Aquarium, Japan, from Dr. Maria Atanassova. 2 Dedicated to 150th anniversary of the discovery of the Periodic Table of chemical elements by Dmitry Mendeleev and 100th anniversary of IUPAC celebrated in 2019 year "By presenting my book to the scientific community, I know there are flaws and shortcomings in it, but I hope that people will be recalled to know that science is immense and that the human mind is limited." Dmitry Ivanovich Mendeleev 3 Preface Inasmuch as ionic liquids (ILs) were reported firstly in the literature in late 1914, there has always been a parallel amid academic scientific research and industrial interest that covers an increasingly diverse range of technological fields, especially for water stable liquid compounds. The proposed overview addresses the investigations of the mutual solubilities between ILs and water attempting to relate them with the chemical nature and structure of these liquid compounds. For the two-phase liquid system, the question arises about how much of each of the pure liquid components dissolves in the other at equilibrium at a given temperature and pressure.