KDIGO Clinical Practice Guideline for Glomerulonephritis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

PATHOLOGY of the RENAL SYSTEM”, I Hope You Guys Like It
ﺑﺴﻢ اﷲ اﻟﺮﺣﻤﻦ اﻟﺮﺣﯿﻢ ھﺬه اﻟﻤﺬﻛﺮة ﻋﺒﺎرة ﻋﻦ إﻋﺎدة ﺗﻨﺴﯿﻖ وإﺿﺎﻓﺔ ﻧﻮﺗﺎت وﻣﻮاﺿﯿﻊ ﻟﻤﺬﻛﺮة زﻣﻼﺋﻨﺎ ﻣﻦ اﻟﺪﻓﻌﺔ اﻟﺴﺎﺑﻘﺔ ٤٢٧ اﻷﻋﺰاء.. ﻟﺘﺘﻮاﻓﻖ ﻣﻊ اﻟﻤﻨﮭﺞ اﻟﻤﻘﺮر ﻣﻦ اﻟﻘﺴﻢ ﺣﺮﺻﻨﺎ ﻓﯿﮭﺎ ﻋﻠﻰ إﻋﺎدة ﺻﯿﺎﻏﺔ ﻛﺜﯿﺮ ﻣﻦ اﻟﺠﻤﻞ ﻟﺘﻜﻮن ﺳﮭﻠﺔ اﻟﻔﮭﻢ وﺳﻠﺴﺔ إن ﺷﺎء اﷲ.. وﺿﻔﻨﺎ ﺑﻌﺾ اﻟﻨﻮﺗﺎت اﻟﻤﮭﻤﺔ وأﺿﻔﻨﺎ ﻣﻮاﺿﯿﻊ ﻣﻮﺟﻮدة ﺑﺎﻟـ curriculum ﺗﻌﺪﯾﻞ ٤٢٨ ﻋﻠﻰ اﻟﻤﺬﻛﺮة ﺑﻮاﺳﻄﺔ اﺧﻮاﻧﻜﻢ: ﻓﺎرس اﻟﻌﺒﺪي ﺑﻼل ﻣﺮوة ﻣﺤﻤﺪ اﻟﺼﻮﯾﺎن أﺣﻤﺪ اﻟﺴﯿﺪ ﺣﺴﻦ اﻟﻌﻨﺰي ﻧﺘﻤﻨﻰ ﻣﻨﮭﺎ اﻟﻔﺎﺋﺪة ﻗﺪر اﻟﻤﺴﺘﻄﺎع، وﻻ ﺗﻨﺴﻮﻧﺎ ﻣﻦ دﻋﻮاﺗﻜﻢ ! 2 After hours, or maybe days, of working hard, WE “THE PATHOLOGY TEAM” are proud to present “PATHOLOGY OF THE RENAL SYSTEM”, I hope you guys like it . Plz give us your prayers. Credits: 1st part = written by Assem “ THe AWesOme” KAlAnTAn revised by A.Z.K 2nd part = written by TMA revised by A.Z.K د.ﺧﺎﻟﺪ اﻟﻘﺮﻧﻲ 3rd part = written by Abo Malik revised by 4th part = written by A.Z.K revised by Assem “ THe AWesOme” KAlAnTAn 5th part = written by The Dude revised by TMA figures were provided by A.Z.K Page styling and figure embedding by: If u find any error, or u want to share any idea then plz, feel free to msg me [email protected] 3 Table of Contents Topic page THE NEPHROTIC SYNDROME 4 Minimal Change Disease 5 MEMBRANOUS GLOMERULONEPHRITIS 7 FOCAL SEGMENTAL GLOMERULOSCLEROSIS 9 MEMBRANOPROLIFERATIVE GLOMERULONEPHRITIS 11 DIABETIC NEPHROPATHY (new) 14 NEPHRITIC SYNDROME 18 Acute Post-infectious GN 19 IgA Nephropathy (Berger Disease) 20 Crescentic GN 22 Chronic GN 24 SLE Nephropathy (new) 26 Allograft rejection of the transplanted kidney (new) 27 Urinary Tract OBSTRUCTION, 28 RENAL STONES 23 HYDRONEPHROSIS -

Defining Natural Antibodies
PERSPECTIVE published: 26 July 2017 doi: 10.3389/fimmu.2017.00872 Defining Natural Antibodies Nichol E. Holodick1*, Nely Rodríguez-Zhurbenko2 and Ana María Hernández2* 1 Department of Biomedical Sciences, Center for Immunobiology, Western Michigan University Homer Stryker M.D. School of Medicine, Kalamazoo, MI, United States, 2 Natural Antibodies Group, Tumor Immunology Division, Center of Molecular Immunology, Havana, Cuba The traditional definition of natural antibodies (NAbs) states that these antibodies are present prior to the body encountering cognate antigen, providing a first line of defense against infection thereby, allowing time for a specific antibody response to be mounted. The literature has a seemingly common definition of NAbs; however, as our knowledge of antibodies and B cells is refined, re-evaluation of the common definition of NAbs may be required. Defining NAbs becomes important as the function of NAb production is used to define B cell subsets (1) and as these important molecules are shown to play numerous roles in the immune system (Figure 1). Herein, we aim to briefly summarize our current knowledge of NAbs in the context of initiating a discussion within the field of how such an important and multifaceted group of molecules should be defined. Edited by: Keywords: natural antibody, antibodies, natural antibody repertoire, B-1 cells, B cell subsets, B cells Harry W. Schroeder, University of Alabama at Birmingham, United States NATURAL ANTIBODY (NAb) PRODUCING CELLS Reviewed by: Andre M. Vale, Both murine and human NAbs have been discussed in detail since the late 1960s (2, 3); however, Federal University of Rio cells producing NAbs were not identified until 1983 in the murine system (4, 5). -

Glomerulonephritis Management in General Practice
Renal disease • THEME Glomerulonephritis Management in general practice Nicole M Isbel MBBS, FRACP, is Consultant Nephrologist, Princess Alexandra lomerular disease remains an important cause Hospital, Brisbane, BACKGROUND Glomerulonephritis (GN) is an G and Senior Lecturer in important cause of both acute and chronic kidney of renal impairment (and is the commonest cause Medicine, University disease, however the diagnosis can be difficult of end stage kidney disease [ESKD] in Australia).1 of Queensland. nikky_ due to the variability of presenting features. Early diagnosis is essential as intervention can make [email protected] a significant impact on improving patient outcomes. OBJECTIVE This article aims to develop However, presentation can be variable – from indolent a structured approach to the investigation of patients with markers of kidney disease, and and asymptomatic to explosive with rapid loss of kidney promote the recognition of patients who need function. Pathology may be localised to the kidney or further assessment. Consideration is given to the part of a systemic illness. Therefore diagnosis involves importance of general measures required in the a systematic approach using a combination of clinical care of patients with GN. features, directed laboratory and radiological testing, DISCUSSION Glomerulonephritis is not an and in many (but not all) cases, a kidney biopsy to everyday presentation, however recognition establish the histological diagnosis. Management of and appropriate management is important to glomerulonephritis (GN) involves specific therapies prevent loss of kidney function. Disease specific directed at the underlying, often immunological cause treatment of GN may require specialist care, of the disease and more general strategies aimed at however much of the management involves delaying progression of kidney impairment. -
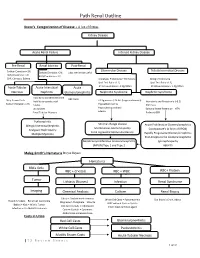
Path Renal Outline
Path Renal Outline Krane’s Categorization of Disease + A lot of Extras Kidney Disease Acute Renal Failure Intrinsic Kidney Disease Pre‐Renal Renal Intrinsic Post‐Renal Sodium Excretion <1% Glomerular Disease Tubulointerstitial Disease Sodium Excretion < 1% Sodium Excretion >2% Labs aren’t that useful BUN/Creatinine > 20 BUN/Creatinine < 10 CHF, Cirrhosis, Edema Urinalysis: Proteinuria + Hematuria Benign Proteinuria Spot Test Ratio >1.5, Spot Test Ratio <1.5, Acute Tubular Acute Interstitial Acute 24 Urine contains > 2.0g/24hrs 24 Urine contains < 1.0g/24hrs Necrosis Nephritis Glomerulonephritis Nephrotic Syndrome Nephritic Syndrome Inability to concentrate Urine RBC Casts Dirty Brown Casts Inability to secrete acid >3.5g protein / 24 hrs (huge proteinuria) Hematuria and Proteinuria (<3.5) Sodium Excretion >2% Edema Hypoalbuminemia RBC Casts Hypercholesterolemia Leukocytes Salt and Water Retention = HTN Focal Tubular Necrosis Edema Reduced GFR Pyelonephritis Minimal change disease Allergic Interstitial Nephritis Acute Proliferative Glomerulonephritis Membranous Glomerulopathy Analgesic Nephropathy Goodpasture’s (a form of RPGN) Focal segmental Glomerulosclerosis Rapidly Progressive Glomerulonephritis Multiple Myeloma Post‐Streptococcal Glomerulonephritis Membranoproliferative Glomerulonephritis IgA nephropathy (MPGN) Type 1 and Type 2 Alport’s Meleg‐Smith’s Hematuria Break Down Hematuria RBCs Only RBC + Crystals RBC + WBC RBC+ Protein Tumor Lithiasis (Stones) Infection Renal Syndrome Imaging Chemical Analysis Culture Renal Biopsy Calcium -

Criteria for the Clinical Use of Intravenous Immunoglobulin in Australia
Standing Council on Health Criteria for the clinical use of intravenous immunoglobulin in Australia Second Edition July 2012 Criteria for the clinical use of intravenous immunoglobulin in Australia Second Edition July 2012 Disclaimer: Important information about this document This document is not a clinical practice guideline. It intends only to provide information about criteria for accessing intravenous immunoglobulin funded under the National Blood Arrangements. Any advice relating to other forms of treatment relevant to the conditions in The Criteria for the clinical use of intravenous immunoglobulin in Australia (the Criteria) have not been subject to a systematic review and should not be relied upon to guide treatment. Patients and doctors should not use this document as a substitute for expert medical guidance and advice. The relevance and appropriateness of information in this document depends, amongst other things, on an accurate diagnosis, the severity of the condition being properly ascertained, the individual response to diagnostic tests and therapies, and other relevant circumstances in each case. The Criteria was first published in 2007 after a systematic review that was completed in mid-2006. Another systematic review, of a limited number of indications, was then undertaken in 2010-11 to update the Criteria. This review resulted in the addition of a small number of indications, removal of a small number of indications and a rewording of a limited number of indications. Inclusion of indications in the Criteria is based where possible upon systematic review of the evidence. In the absence of published evidence, information and access criteria are based on clinical advice provided to the development group by clinical colleges, clinical societies and individual experts. -

Nephrology Clinical Undergraduate Training
School of Medicine Nephrology clinical undergraduate training All care is taken to ensure that the information in this handbook is correct at the time of going to print. Handbook Version 5.0 Date of Origin: March 2019 Contents OBJECTIVES OF ATTACHMENT ................................................................................................... 3 TEACHING STRUCTURE: 3RD MEDICAL YEAR CLINICAL MEDICINE ATTACHMENTS .......................... 3 PROPOSED ATTACHMENT TIMETABLE: ................................................................................................ 5 READING LIST AND WEBSITES ............................................................................................................. 6 2 3rd Medical Year SPECIALTY: Nephrology - www.tcd.ie/medicine/thkc/education/ CONSULTANT: Prof G. Mellotte, Prof C Wall, Dr P Lavin, Dr B Griffin, Prof M Little HOSPITAL: Trinity Health Kidney Centre, Tallaght Hospital YEAR OF COURSE: 3 OBJECTIVES OF ATTACHMENT During this attachment, a student is expected to understand: - The clinical presentation of renal disease, e.g., proteinuria, hypertension, haematuria and uraemia. - Normal regulation of body water and sodium by the RAAS and ADH and how abnormalities give rise to changes in water and sodium homeostasis - Normal values of electrolytes in blood and urine and the clinical sequelae of a derangement in these. - A basic understanding of the following conditions: a. Acute kidney injury (pre-renal, post renal or intrinsic renal) b. Chronic kidney disease, focusing on diabetic nephropathy c. Glomerulonephritis: nephrotic / nephritic syndrome d. Myeloma and the kidney - The management of acute and chronic renal failure, including preparation for dialysis. - Impact of renal failure on drug handling. Be able to: - Take a full and appropriate current and past medical history. - Construct a synopsis or problem list based on the clinical assessment of a patient - Discuss the range of clinical investigations available and understand how they may be used to inform the differential diagnosis. -

16 the Kidney J
16 The Kidney J. Charles Jennette FPO FPO FPO FPO CONGENITAL ANOMALIES IgA Nephropathy (Berger Disease) Renal Agenesis Anti-Glomerular Basement Membrane Ectopic Kidney Glomerulonephritis Horseshoe Kidney ANCA Glomerulonephritis Renal Dysplasia VASCULAR DISEASES CONGENITAL POLYCYSTIC KIDNEY DISEASES Renal Vasculitis Autosomal Dominant Polycystic Kidney Disease Hypertensive Nephrosclerosis (Benign Nephrosclerosis) (ADPKD) Malignant Hypertensive Nephropathy Autosomal Recessive Polycystic Kidney Disease Renovascular Hypertension (ARPKD) Thrombotic Microangiopathy Nephronophthisis–Medullary Cystic Disease Cortical Necrosis ACQUIRED CYSTIC KIDNEY DISEASE DISEASES OF TUBULES AND INTERSTITIUM GLOMERULAR DISEASES Acute Tubular Necrosis (ATN) Nephrotic Syndrome Pyelonephritis Nephritic Syndrome Analgesic Nephropathy Glomerular Inflammation and Immune Mechanisms Drug-Induced (Hypersensitivity) Acute Tubulointerstitial Minimal-Change Glomerulopathy Nephritis Focal Segmental Glomerulosclerosis (FSGS) Light-Chain Cast Nephropathy Membranous Glomerulopathy Urate Nephropathy Diabetic Glomerulosclerosis RENAL STONES (NEPHROLITHIASIS AND Amyloidosis UROLITHIASIS) Hereditary Nephritis (Alport Syndrome) OBSTRUCTIVE UROPATHY AND HYDRONEPHROSIS Thin Glomerular Basement Membrane Nephropathy RENAL TRANSPLANTATION Acute Postinfectious Glomerulonephritis MALIGNANT TUMORS OF THE KIDNEY Type I Membranoproliferative Glomerulonephritis Wilms’ Tumor (Nephroblastoma) Type II Membranoproliferative Glomerulonephritis Renal Cell Carcinoma (RCC) (Dense Deposit Disease) -

Jemds.Com Original Research Article
Jemds.com Original Research Article CLINICAL PROFILE AND SHORT-TERM OUTCOME OF ACUTE NEPHRITIC SYNDROME IN CHILDREN Surya Kandashamparambil Kamalakarababu1, Ansu Sam2, Sajini Varghese3 1Assistant Professor, Department of Paediatrics, Government Medical College, Kottayam. 2Senior Resident, Department of Paediatrics, Government Medical College, Kottayam. 3Assistant Professor, Department of Paediatrics, Government Medical College, Kottayam. ABSTRACT BACKGROUND Glomerulonephritis generally presents as a constellation of findings that includes haematuria, proteinuria and oedema. Poststreptococcal glomerulonephritis is the commonest form of acute glomerulonephritis in developing countries. Objectives- 1. To study the clinical profile of acute glomerulonephritis. 2. To study the different clinical laboratory parameters at admission and at 8 weeks of onset of illness. MATERIAL AND METHODS This is a prospective, descriptive study conducted in a tertiary care teaching hospital in South India from January 2016-December 2016. Data regarding the clinical features, laboratory parameters, treatment were collected. All these patients were further followed up at 8 weeks and descriptive analysis done. RESULTS The most common cause of glomerulonephritis in the study group was poststreptococcal glomerulonephritis (42.9%) followed by drug-induced nephropathy (28.6%). The most common complaints were haematuria, facial puffiness, decreased urine output and oedema. Hypertension was found only in 14.3% of study group. 4.8% of patients had nephrotic range of proteinuria. The most common infection preceding was Pyoderma (61.5%) in case of PSGN. High ASO titre was seen commonly with pharyngitis. 45.2% patients had decreased Serum C3 at diagnosis. All the patients were treated conservatively. At 8 weeks followup, haematuria was persisting in 28.6% patients. Only 2 patients had persistently low serum C3 and single patient had high creatinine. -

I M M U N O L O G Y Core Notes
II MM MM UU NN OO LL OO GG YY CCOORREE NNOOTTEESS MEDICAL IMMUNOLOGY 544 FALL 2011 Dr. George A. Gutman SCHOOL OF MEDICINE UNIVERSITY OF CALIFORNIA, IRVINE (Copyright) 2011 Regents of the University of California TABLE OF CONTENTS CHAPTER 1 INTRODUCTION...................................................................................... 3 CHAPTER 2 ANTIGEN/ANTIBODY INTERACTIONS ..............................................9 CHAPTER 3 ANTIBODY STRUCTURE I..................................................................17 CHAPTER 4 ANTIBODY STRUCTURE II.................................................................23 CHAPTER 5 COMPLEMENT...................................................................................... 33 CHAPTER 6 ANTIBODY GENETICS, ISOTYPES, ALLOTYPES, IDIOTYPES.....45 CHAPTER 7 CELLULAR BASIS OF ANTIBODY DIVERSITY: CLONAL SELECTION..................................................................53 CHAPTER 8 GENETIC BASIS OF ANTIBODY DIVERSITY...................................61 CHAPTER 9 IMMUNOGLOBULIN BIOSYNTHESIS ...............................................69 CHAPTER 10 BLOOD GROUPS: ABO AND Rh .........................................................77 CHAPTER 11 CELL-MEDIATED IMMUNITY AND MHC ........................................83 CHAPTER 12 CELL INTERACTIONS IN CELL MEDIATED IMMUNITY ..............91 CHAPTER 13 T-CELL/B-CELL COOPERATION IN HUMORAL IMMUNITY......105 CHAPTER 14 CELL SURFACE MARKERS OF T-CELLS, B-CELLS AND MACROPHAGES...............................................................111 -

A Guide for Primary Care Physicians
OPTIMUM PHYSICIAN ALLIANCE CONTACT PO Box 90 MERI NOTARO Buffalo, New York opawny.com 14240 1-844-769-5879 OPA 2017 NETWORK + INCENTIVE GUIDE Prepared For: Date Issued: A GUIDE FOR 10.31.2016 PRIMARY CARE PHYSICIANS Valid To: 12.31.2017 OPA PHYSICIAN PARTNERS Table of Contents CONTENTS 1 2 3 Take a look at what you'll find inside this guide. INTRODUCTION TIERING PROGRAM STANDARD INCENTIVE OPA Overview 3 OPA Primary Care 12 Standard Incentive Overview 17 Physician Tiering Overview 2017 Network Standards 5 Primary Care Metrics 19 2017 PCP 13 OPA Resources 7 Tiering Structure Pediatric Care Metrics 22 Network Incentive and 9 Standard Incentive Metrics 23 PCP Tiering Overview 4 5 6 CENTERS OF EXCELLENCE PRACTICE OPTIMIZATION APPENDICES PROGRAM (POP) Centers of Excellence 25 Practice Optimization 31 Standard Incentive 45 Overview Program (POP) Guidelines Code Lists Activity Based 27 Sample POP Application 39 Performance Measures Utilization Based 28 Performance Measures Centers of Excellence Metrics 29 1 OPTIMUM PHYSICIAN ALLIANCE OPTIMUM PHYSICIAN ALLIANCE 2 [ the platform that simplifies the practice of medicine ] Optimum Physician Alliance (OPA), formed in 2012, is the platform that simplifies the practice of medicine. It consists of approximately 600 physicians in the areas of primary and specialty care, Organization located mainly in Erie and Niagara counties. OPA is committed to promoting prac- meaningful engagement among healthcare tice transformation, with a patient-centered partners. OPA provides key resources to reduce Overview approach, and understands the importance administrative burdens and offers incentive pro- of leveraging healthcare data to support high grams designed to promote the type of practice quality outcomes for patients. -

Safety and Tolerability of Plasma Exchange and Immunoadsorption in Neuroinflammatory Diseases
Journal of Clinical Medicine Article Safety and Tolerability of Plasma Exchange and Immunoadsorption in Neuroinflammatory Diseases Johannes Dorst 1,* , Frank Fillies 1, Jens Dreyhaupt 2 , Makbule Senel 1 and Hayrettin Tumani 1 1 Department of Neurology, University of Ulm, 89081 Ulm, Germany; frank.fi[email protected] (F.F.); [email protected] (M.S.); [email protected] (H.T.) 2 Institute for Epidemiology and Medical Biometry, University of Ulm, 89081 Ulm, Germany; [email protected] * Correspondence: [email protected] Received: 13 August 2020; Accepted: 3 September 2020; Published: 5 September 2020 Abstract: Plasma exchange (PE) and immunoadsorption (IA) are frequently used for treatment of various autoimmune-mediated neurological diseases, including multiple sclerosis (MS), chronic inflammatory demyelinating polyneuropathy (CIDP), and Guillain–Barré syndrome (GBS). Although both methods are generally regarded as well-tolerated treatment options, evidence for safety and tolerability is low for most indications and largely relies on small case series. In this study, we retrospectively analysed adverse events (AEs) and laboratory changes in 284 patients with various neurological indications who received either PE (n = 65, 113 cycles) or IA (n = 219, 435 cycles) between 2013 and 2020 in our Neurology department. One standard treatment cycle for PE as well as IA consisted of five treatments on five consecutive days. During every treatment, the 2.0–2.5-fold individual plasma volume (PV) was treated in IA, while in PE, the 0.7-fold individual PV was replaced by human albumin solution. Overall, both methods showed an excellent safety profile; no deaths of life-threatening adverse events were recorded. -

Treatment of Donor-Specific Antibody-Mediated Graft Rejection by Immunochemotherapy, Third-Party DLI, Plasmapheresis and Immunoa
Bone Marrow Transplantation (2015) 50, 613–614 © 2015 Macmillan Publishers Limited All rights reserved 0268-3369/15 www.nature.com/bmt LETTER TO THE EDITOR Treatment of donor-specific antibody-mediated graft rejection by immunochemotherapy, third-party DLI, plasmapheresis and immunoadsorption Bone Marrow Transplantation (2015) 50, 613–614; doi:10.1038/ B cells, we first administered immunochemotherapy according to bmt.2014.321; published online 26 January 2015 a modified fludarabine, cytarabine, rituximab (FCR) scheme (rituximab 3 × 375 mg/m2 days +27 to +30, cyclophosphamide 2 fl 2 fi 3 × 300 mg/m and udarabine 3 × 30 mg/m both days +30 to In allogenic SCT, patient-derived donor-speci c HLA antibody +32). Most prophylactic desensitization protocols used irradiated (DSA)-mediated graft rejection is a rare, but serious complication. 1 lymphocyte infusions (DLI) or platelet infusions from the original Gergis et al. recently described a new therapeutic approach to donor or infusion of surrogate platelets.2 In our patient, no DLI or reduce pre-existing DSA before allogeneic transplantation to donor platelets were available, as this donor was prepared for the enhance the likelihood of engraftment in 10 patients. They second donation. In this special situation, we took advantage of combined pretransplant extracorporeal antibody elimination by the fact that the haploidentical son carried the same mismatched plasmapheresis with immunochemotherapy (rituximab, bortezo- A2 antigen as the donor (Table 1). We hence isolated a total of mib and/or intravenous immunglobuline (i.v.IG)) and thereby 1.9 × 108 CD3+ lymphocytes/kg from the haploidentical son and achieved substantial reduction in DSA titers with sustained infused these cells after irradiation in three fractions on days +32 engraftment in nine patients.