Roles of Carbohydrates and Proteins in the Staling Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ethnic and Traditional Iranian Breads: Different Types, and Historical and Cultural Aspects
J Ethn Foods - (2017) 1e7 Contents lists available at ScienceDirect Journal of Ethnic Foods journal homepage: http://journalofethnicfoods.net Original article Ethnic and traditional Iranian breads: different types, and historical and cultural aspects * Vahid Mohammadpour Karizaki Chemical Engineering Department, Quchan University of Advanced Technology, Quchan, Iran article info abstract Article history: Background: Bread making has a long history in Iran. Because of the inseparable relationship between Received 21 December 2016 Iranian people and bread, an increasingly wide variety of this healthy and nutritious food is prepared and Received in revised form consumed throughout the country. The present work aims at documenting and providing information 14 January 2017 about breads of Iranian cuisine. Accepted 20 January 2017 Methods: The required information was obtained via a direct face-to-face questionnaire-based survey Available online xxx among housewives, domestic people, and Iranian bakers. The statistical society was selected by random sampling among people from the top eight most populous cities in the country. Keywords: bread Results: More than 30 types of ethnic and traditional bread of Iranian cuisine are introduced in two main fi ethnic food categories: the rst group includes breads that are consumed all around the country, and the second Iran group consists of those that are prepared in special regions, or by ethnic groups. Conclusion: The historical and cultural aspects of the Iranian foods showed that bread is the most common and popular food in the country. © 2017 Korea Food Research Institute. Published by Elsevier B.V. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/). -

2020 Annual Recipe SIP.Pdf
SPECIAL COLLECTOR’SEDITION 2020 ANNUAL Every Recipe from a Full Year of America’s Most Trusted Food Magazine CooksIllustrated.com $12.95 U.S. & $14.95 CANADA Cranberry Curd Tart Display until February 22, 2021 2020 ANNUAL 2 Chicken Schnitzel 38 A Smarter Way to Pan-Sear 74 Why and How to Grill Stone 4 Malaysian Chicken Satay Shrimp Fruit 6 All-Purpose Grilled Chicken 40 Fried Calamari 76 Consider Celery Root Breasts 42 How to Make Chana Masala 77 Roasted Carrots, No Oven 7 Poulet au Vinaigre 44 Farro and Broccoli Rabe Required 8 In Defense of Turkey Gratin 78 Braised Red Cabbage Burgers 45 Chinese Stir-Fried Tomatoes 79 Spanish Migas 10 The Best Turkey You’ll and Eggs 80 How to Make Crumpets Ever Eat 46 Everyday Lentil Dal 82 A Fresh Look at Crepes 13 Mastering Beef Wellington 48 Cast Iron Pan Pizza 84 Yeasted Doughnuts 16 The Easiest, Cleanest Way 50 The Silkiest Risotto 87 Lahmajun to Sear Steak 52 Congee 90 Getting Started with 18 Smashed Burgers 54 Coconut Rice Two Ways Sourdough Starter 20 A Case for Grilled Short Ribs 56 Occasion-Worthy Rice 92 Oatmeal Dinner Rolls 22 The Science of Stir-Frying 58 Angel Hair Done Right 94 Homemade Mayo That in a Wok 59 The Fastest Fresh Tomato Keeps 24 Sizzling Vietnamese Crepes Sauce 96 Brewing the Best Iced Tea 26 The Original Vindaloo 60 Dan Dan Mian 98 Our Favorite Holiday 28 Fixing Glazed Pork Chops 62 No-Fear Artichokes Cookies 30 Lion’s Head Meatballs 64 Hummus, Elevated 101 Pouding Chômeur 32 Moroccan Fish Tagine 66 Real Greek Salad 102 Next-Level Yellow Sheet Cake 34 Broiled Spice-Rubbed 68 Salade Lyonnaise Snapper 104 French Almond–Browned 70 Showstopper Melon Salads 35 Why You Should Butter- Butter Cakes 72 Celebrate Spring with Pea Baste Fish 106 Buttermilk Panna Cotta Salad 36 The World’s Greatest Tuna 108 The Queen of Tarts 73 Don’t Forget Broccoli Sandwich 110 DIY Recipes America’s Test Kitchen has been teaching home cooks how to be successful in the kitchen since 1993. -
Shelf-Life Guide
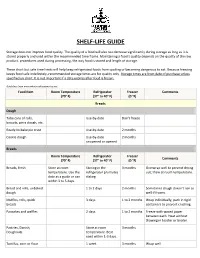
SHELF-LIFE GUIDE Storage does not improve food quality. The quality of a food will also not decrease significantly during storage as long as it is stored properly and used within the recommended time frame. Maintaining a food’s quality depends on the quality of the raw product, procedures used during processing, the way food is stored and length of storage. These short but safe time limits will help keep refrigerated foods from spoiling or becoming dangerous to eat. Because freezing keeps food safe indefinitely, recommended storage times are for quality only. Storage times are from date of purchase unless specified on chart. It is not important if a date expires after food is frozen. Guidelines from www.whatscookingamerica.net Food Item Room Temperature Refrigerator Freezer Comments (70° F) (37° to 40° F) (0 °F) Breads Dough Tube cans of rolls, Use-by-date Don't freeze biscuits, pizza dough, etc. Ready-to-bake pie crust Use-by-date 2 months Cookie dough Use-by-date 2 months unopened or opened Breads Room Temperature Refrigerator Freezer Comments (70° F) (37° to 40° F) (0 °F) Breads, fresh Store at room Storing in the 3 months Overwrap well to prevent drying temperature. Use the refrigerator promotes out; thaw at room temperature. date as a guide or use staling. within 3 to 5 days. Bread and rolls, unbaked 1 to 2 days 2 months Sometimes dough doesn't rise as dough well if frozen. Muffins, rolls, quick 3 days 1 to 2 months Wrap individually, pack in rigid breads containers to prevent crushing. -

Starch Chemistry Staling Causes and Effects
VOLUME 3 /NUMBER 9 Bread Staling Practical technology from Lallemand Inc. Starch Staling Causes and Effects Chemistry TALING REFERS T O the undesirable wrapped bread tastes dry because water has D-glucose is the basic building block of changes (other than microbial spoil- migrated from the crumb to the crust and starch. Its chemical model is a hexagon Sage) that take place between the time from the starch to the gluten. made up of one oxygen atom, five carbon bread is baked and consumed. Understand- Crust softening in wrapped bread is atoms (numbered 1 through 5), and two ing the different aspects of staling and the caused by an increase in moisture from about forms ( alpha- and beta- ) depending on its factors that affect them can help bakers 12 to 28 percent. This changes the dry, crisp, structure at position 1: make better decisions about their formu - pleasant texture of fresh crust into the soft, las, ingredients, processes, and packaging. leathery, unpleasant texture of stale crust. 6CH OH 6CH OH 2 2 Flavor losses and changes occur as 5 O 5 O HH H HOHH ASPECTS OF STALING some flavor components diminish faster 1( ) 1 4 OH H α 4 OH H (β) Crumb firming is caused by changes in than others. The taste o f fresh bread is usu - HO OH HO H 3 2 3 2 starch structure. The starch in wheat flour ally a combination of sweet, salty, and H OH H OH is made up of straight and branched chains slightly sour, but with age the sweet and alpha-glucose beta-glucose contained in granules. -

“Give Us This Day Our Daily Bread”
“Give us this day our daily bread” Rosalba Gentile Abstract Bread is a universal and ancient staple that in Italy is regarded as the symbol of both food and national unity. Nonetheless, the great variety of breads dating back to Roman times and still available in the country recalls the political fragmentation of pre-Unification Italy. Furthermore, as it is essentially a cultural object, bread is closely intertwined with the social structure of Italian society whose cultural and dietary shifts, however, have adversely affected bread production and consumption. The latter, moreover, is also linked to the so- called diseases of civilisation that actually constitute public health problems. The political aspects of bread analysed in this paper mainly pertain to the national and European legislation which establishes the characteristics and names of Italian bread, and to the gradual disappearance of artisan bakery that does not seem to attract the younger generations. The relationship between bread and environment, on the other hand, shows how climate change, pesticides, and fertilisers negatively impact on wheat production. Additionally, bread-making technology and the controversial issue relating to GM crops highlight the unavoidable role of biotechnology in the future nutritional scenario. A scenario that is likely to be dominated by healthy foods such as gluten-free, salt- free and functional breads. Keywords: bread, Italy, cultural object, environment, bread-technology. Introduction Bread has been widely recognised as one of the most ancient and basic foods of mankind;1 a daily staple that has significantly helped to form the foundations of civilisation2 and which still lies at the heart of contemporary society.3 This basically allows to view bread as a sort of “prism” that is apt to imbibe, refract, and eventually unify a great variety of cultural events, thus creating a common and coherent language;4 i.e., a ‘sensory language’ permitting a mutual comprehension between 1 Cauvain, S.P., 1998. -

Impact of Baking Bed and Baking Temperature on Staling of Sangak Bread
J. Agr. Sci. Tech. (2015) Vol. 17: 375-386 Impact of Baking Bed and Baking Temperature on Staling of Sangak Bread ∗ L. Izadi Najafabadi 1 , N. Hamdami 1, A. Le-Bail 2, J. Y. Monteau 2, and J. Keramat 1 ABSTRACT The mode of heat and mass transfer during baking of bakery products is an important factor determining the quality of the final product. The heating rate could alter the starch properties during gelatinization and affect the quality of products after baking and during aging. Sangak is a kind of Iranian flat bread baked on the hot pebble gravels. The aim of this study was to evaluate the impacts of baking beds (gravel and metal beds) and baking temperatures (280, 310 and 340°C) on staling of Sangak bread during storage at 20°C. The mean value of the measured heating rate of bread baked on gravel bed was higher than that in bread baked on metal bed. In the case of baking on gravel bed, unlike baking on the metal bed, the increase of baking temperature had no significant effect on all quality parameters (moisture content, firmness and freezable water of breads, etc). Recrystallization of amylopectin seemed not to be related to the baking condition. During aging, the firmness of bread baked on gravel bed was significantly lower than that for bread baked on the metal bed and baking at higher temperature and shorter time resulted in the increment of moisture content and the decrease of firmness. As a consequence, baking on gravels and higher baking temperature increased the heating rate, which led to reduction of the staling kinetic of Sangak bread. -

1 CHAPTER ONE INTRODUCTION Cereal-Based Foods Play An
CHAPTER ONE INTRODUCTION Cereal-based foods play an important role as a source of dietary energy and nutrients in human nutrition. The use of microorganisms by humans has a long tradition. Besides beer and wine production, bread making is one of the oldest arts known to man. For example, Egypt, Greece and Italy were early places of discovery of leavened breads (Kulp and Lorenz, 2003). Wheat falls under the grass family Gramineae, which include the cereal grains. Other popular cereal grains that fall under this category are corn, rice, barley, oats, sorghum and pearl millet. All of this can be milled into flour, but only wheat flour has the ability to be transformed into glutinous dough, together with water and other ingredients. The uniqueness of wheat flour to be transformed into a cohesive, viscoelastic mass is due to the hydration of gluten proteins in the wheat together with the application of mixing energy. After the dough is formed, the gluten structure entraps the gasses produced during the fermentation stage. This allows the dough mass to expand and to be baked into a soft, light and palatable product, which is known as bread (September, 2007). Fermented foods, particularly those produced under controlled conditions, have a good record of safety and are implicated in outbreaks of diseases relatively infrequently (Adams and Nout, 2001). Bread is a staple foodstuff made and eaten in most countries around the world (Owens, 2001). Bread is the most popular yeast leaved product made from wheat flour. The bread making process is one of the oldest applications of biotechnology. -

Resistant Starch in the Italian Diet*
Downloaded from British Journal of Nutrition (1998), 80, 333–341 333 https://www.cambridge.org/core Resistant starch in the Italian diet* Furio Brighenti†, M. Cristina Casiraghi and Cristina Baggio . IP address: Department of Food Science & Microbiology, Nutrition Unit, University of Milan, Via Celoria no. 2, 20133 Milano, Italy (Received 16 October 1997 – Revised 9 April 1998 – Accepted 12 May 1998) 170.106.34.90 Resistant starch (RS) has been defined as the sum of starch and starch-degradation products that reach the human large intestine (Champ, 1994), and it is now regarded as a sub-fraction of starch , on with a positive impact on colonic welfare and lipid metabolism. An early estimate of the RS 03 Oct 2021 at 02:49:27 intake in Europe gave an average value of approximately 4 g/d (Dyssler & Hoffem, 1994a). However, since no information is available for Italy, the aim of the present study was to estimate the intake of RS in the Italian diet by direct analysis of RS in a range of typical foods representing the main sources of starch intake in the country. The selection of representative foods and of food consumption data were based on published results of the National Food Consumption Study conducted during the 1980s by the National Institute of Nutrition on 10 000 households, using , subject to the Cambridge Core terms of use, available at weighed-food records plus inventory methodologies (Saba et al. 1990; Turrini et al. 1991). Three main groups of foods were considered: cereals (pasta, rice, bread and bread products, and pastries), potatoes, legumes. -

Feast on Formulation Inspiration
[INGREDIENTS] PREVIEW by Karen Nachay Feast on Formulation Inspiration ooking for some formulation inspiration? It will To illustrate how ingredients behave in be available in abundance at the 2013 IFT Annual formulations, ingredient suppliers will invite LMeeting & Food Expo®. Many of the hundreds of attendees to sample prototypes like colorful ingredient suppliers who will be on hand will intro- cereal, rich and creamy Greek yogurt, bev- duce brand new ingredients. Others will feature erages in a variety of on-trend flavors, crisp their most successful ingredients to help food tech- crackers, chewy cookies, gluten-free nologists solve formulation challenges. Several will options, granola bars formulated with whole present technical sessions and poster sessions to grains, meat accented with flavorful sea- explain the latest research and sound science soning blends, dairy desserts like ice cream, about both current and novel ingredients. This and so much more. Check out the “Taste the ingredients preview section provides an overview Expo” section of the IFT Annual Meeting & of some of the ingredient innovation that will be on Food Expo mobile app and read the digital display in Chicago this summer. show daily, IFTLive, each day to learn about The Annual Meeting & Food Expo will offer some of the ingredient suppliers featuring attendees a chance to learn about texturizing solu- product concepts available for sampling. tions, formulating with whole grains, sweetener The IFT Food Expo will be a great oppor- solutions, lowering sodium in foods, ingredients for tunity to interact with experts, especially gluten-free applications, global cuisines, improving those responsible for developing some of product performance, and much more. -

Sustainability Assessment of Food Supply Chains
Galli et al. Agricultural and Food Economics (2015) 3:21 DOI 10.1186/s40100-015-0039-0 RESEARCH Open Access Sustainability assessment of food supply chains: an application to local and global bread in Italy Francesca Galli1*, Fabio Bartolini1, Gianluca Brunori1, Luca Colombo2, Oriana Gava1, Stefano Grando2 and Andrea Marescotti3 * Correspondence: [email protected] Abstract This paper has been selected as a ’ ’ best paper of the 51th SIDEA Over the 2000s , consumers food purchases have been increasingly informed by Conference in Benevento (18-20 supply chain-related issues, with growing concerns about the sustainability of chains September 2014). It has been differing for their geographical scope. As a result, short food supply chains and accepted for publication in this ’ journal following the usual revision local food systems have risen to policymakers and food chain stakeholders process. attention as more sustainable alternatives to mainstream food networks. However, 1Department of Agriculture, Food associatingfoodchain’s geographical scope and sustainability performance may and Environment, University of Pisa, via del Borghetto 80, 56124 Pisa, not be straightforward. This paper aims at shedding lights on the connection Italy between geographical scope and sustainability by comparing and discussing 19 Full list of author information is attributes owing to different sustainability dimensions. The analysis anchors on the available at the end of the article wheat-to-bread chain, due to its global relevance. Bread is a worldwide staple food and wheat is (generally) a commodity traded globally. However, wheat processing often occurs locally and baking is influenced by local heritage and consumption patterns, particularly in the EU and in Italy, where gastronomy is culturally embedded. -

Free Ebooks ==> Www
Free ebooks ==> www.Ebook777.com www.Ebook777.com Free ebooks ==> www.Ebook777.com Bakers’ secrets for making amazing long-rise loaves at home Slow Dough REAL BREAD CHRIS YOUNG AND THE BAKERS OF THE REAL BREAD CAMPAIGN www.Ebook777.com For Marika Contents Slow Dough: Real Bread ISBN: 978-1-84899-737-0 Chris Young 10 9 8 7 6 5 4 3 2 1 First published in the UK and USA in 2016 by Typeset in Brandon Grotesque and Adobe Jenson Pro 4 Foreword Nourish, an imprint of Watkins Media Limited 19 Cecil Court Colour reproduction by XY Digital 6 The Fight for Better Bread London WC2N 4EZ Printed in China 10 Real Slow Bread [email protected] Publisher’s note: 16 Terms and Techniques While every care has been taken in compiling the recipes for Copyright © Watkins Media Limited 2016 this book, Watkins Media Limited, or any other persons who 23 Equipment Text copyright © Chris Young 2016 have been involved in working on this publication, cannot Photography copyright © Watkins Media Limited 2016 26 Ingredients accept responsibility for any errors or omissions, inadvertent The right of Chris Young to be identified as the Author of or not, that may be found in the recipes or text, nor for 32 Troubleshooting this text has been asserted in accordance with the Copyright, any problems that may arise as a result of preparing one of 35 Pre-Ferment Designs and Patents Act of 1988. these recipes. If you are pregnant or breastfeeding or have any special dietary requirements or medical conditions, it is 63 Long Ferment All rights reserved. -

The Ancient Tradition of Bread Baking Depends on a Cascade of Chemical Reactions. As Scientists Have Unravelled This Complex
Bread chemistry On the rise The ancient tradition of bread baking depends on a cascade of chemical reactions. As scientists have unravelled this complex chemistry, they have also found myriad ways to modify the process, say Bryan Reuben and Tom Coultate The usually quiet world of bread Protein is a key constituent of has been disturbed recently by In short the wheat flour used for bread both economic and technological A series of chemical production. An essential component changes. Wild fluctuations in the reactions take place of the baked bread’s final structure, price of wheat in the past two throughout the process it is a favourite target for scientists years have made life difficult for of turning flour into looking to intervene in the baking the milling and baking industries bread process. Flour used for most modern and, at one point, the percentage Bread owes much of bread-making in the UK contains at increase in price of bread in the UK its structure to gluten least 12 per cent protein, almost all was greater than at any time since proteins – a key target of this the so-called gluten proteins deregulation in the 1950s. The credit for chemists looking to that would provide nitrogen for the crunch has pushed people towards intervene in the process growth of the germinating wheat bread as a replacement for more Traditional bread seedling. expensive foods – in the UK, the making is a slow process, As the baker mixes flour with annual quantity of bread sold up to prompting scientists to water at the start of the bread- April 2009 showed the first increase develop ‘no time’ doughs making process, these proteins for 35 years.