The Ancient Tradition of Bread Baking Depends on a Cascade of Chemical Reactions. As Scientists Have Unravelled This Complex
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

5-Hydroxymethylfurfural Formation in Bread As a Function of Heat Treatment Intensity: Correlations with Browning Indices
foods Article 5-Hydroxymethylfurfural Formation in Bread as a Function of Heat Treatment Intensity: Correlations with Browning Indices Gabriella Giovanelli and Carola Cappa * Dipartimento di Scienze per gli Alimenti, la Nutrizione e l’Ambiente, Università degli Studi di Milano, Via G. Celoria, 2-20133 Milano, Italy; [email protected] * Correspondence: [email protected]; Tel.: +39-025-0319-179; Fax: +39-503-19-190 Abstract: 5-hydroxymethylfurfural (HMF) is formed during bread baking as a Maillard reaction product (MRP); it can exert toxicity and it is regarded as a potential health risk because of its high consumption levels in western diets. The aim of this study was to evaluate HMF formation in bread as a function of heat treatment intensity (HTI) and to investigate correlations between HMF and easily detectable browning indices. White breads were baked at 200 ◦C and 225 ◦C for different baking times for a total of 24 baking trials. Browning development was evaluated by reflectance colorimetric and computer vision colour analysis; MRP were quantified spectrophotometrically at 280, 360 and 420 nm and HMF was determined by HPLC. HMF concentrations varied from 4 to 300 mg/kg dw. Colour indices (100–L*) and Intensity mean resulted significantly correlated between each other (r = −0.961) and with MRP (r ≥ 0.819). The effects of the different HTI were visualized by principal component analysis and the data were used to evaluate the best fitting regression models between HMF concentration and other browning indices, obtaining models with high correlation coefficients (r > 0.90). Citation: Giovanelli, G.; Cappa, C. Keywords: bread; Maillard reaction products; HMF; browning; colour; regression models 5-Hydroxymethylfurfural Formation in Bread as a Function of Heat Treatment Intensity: Correlations with Browning Indices. -

Bakery and Confectionary HM-302 UNIT: 01 HISTORICAL BACKGROUND of BAKING
Bakery and Confectionary HM-302 UNIT: 01 HISTORICAL BACKGROUND OF BAKING STRUCTURE 1.1 Introduction 1.2 Objective 1.3 Historical Background of Baking 1.4 Introduction to Large, Small Equipments and Tools 1.5 Wheat 1.5.1 Structure of Wheat 1.5.2 Types of Flour 1.5.3 Composition Of Flour 1.5.4 WAP of Flour 1.5.5 Milling of Wheat 1.5.6 Differences Between Semolina, Whole Wheat Flour And Refined Flour 1.5.7 Flour Testing 1.6 Summary 1.7 Glossary 1.8 Reference/Bibliography 1.9 Terminal Questions 1.1 INTRODUCTION BREAD!!!!…….A word of many meanings, a symbol of giving, one food that is common to so many countries….but what really is bread ????. Bread is served in various forms with any meal of the day. It is eaten as a snack, and used as an ingredient in other culinary preparations, such as sandwiches, and fried items coated in bread crumbs to prevent sticking. It forms the bland main component of bread pudding, as well as of stuffing designed to fill cavities or retain juices that otherwise might drip out. Bread has a social and emotional significance beyond its importance as nourishment. It plays essential roles in religious rituals and secular culture. Its prominence in daily life is reflected in language, where it appears in proverbs, colloquial expressions ("He stole the bread from my mouth"), in prayer ("Give us this day our daily bread") and in the etymology of words, such as "companion" (from Latin comes "with" + panis "bread"). 1.2 OBJECTIVE The Objective of this unit is to provide: 1. -

Harvesting-Traditions-1-47-Rough-In
HARVESTING TRADITIONS HARVESTING TRADITION Written and produced by BENJAMIN LESTER Writing Edited by Peter Kobel and Kate Henessy Photography, Layout, and design by Benjamin Lester www.localgrain.org In loving Memory of My Father, James Matthew Lester and For My Mother, Margaret Lester Copyright Farm Feast 2018 and All Rights Reserved my step Mother Nancy Lester My three wonderful, truly good parents Words can’t sumate how fortunate I have been but hopefully this book can. Table of Contents 1. Introduction 2. Culling The Harvest 3. Reverence, Connection and Gratitutude 4. The Essential Storm 5.Our Great Grains 6. Heritage Wheat 7.Milling 8. Hielroom Corn 9. Measurement 10. Paddy Rice 11. Storage 12.Beans 13.Temperature 14.Oats, buckwheat 15. fermentation 16.Heritage Grain Brewing 17. rye 18.Timing ACKNOWLEDGEMENTS This book would not be possible without the tireless work and dedication of our farmers. Alan Zuchowski, Stan, Simon, and Abbie White, Sara and Matt Williams, (etc...) My company My Family Community, members, and supporters Hosts Capsicum ovatum DC. Capsicum petenense Standl. Capsicum pomiferum Mart. ex Steud. Capsicum purpureum Vahl ex Hornem. Capsicum pyramidale Mill. Capsicum quitense Willd. ex Roem. & Schult. Capsicum silvestre Vell. Capsicum sphaerium Willd. Capsicum tetragonum Mill. Capsicum tomatiforme Fingerh. ex Steud. Capsicum torulosum Hornem. Capsicum tournefortii Besser Capsicum ustulatum Paxton “The Most Wonderful Story I know is, perhaps, that this bread, thousands of years old though it is, is not yet finished in the baking. Botanist, famer, miller, and baker are stillexperimenting with it. The entire story of bread goes very deep-its social and technical, religious, political, and scientific story” H.E Jacob “Six Thousand Years of Bread” The Richness of Connection We absorb the most valuable lessons from our parents through how they live their lives. -

Ethnic and Traditional Iranian Breads: Different Types, and Historical and Cultural Aspects
J Ethn Foods - (2017) 1e7 Contents lists available at ScienceDirect Journal of Ethnic Foods journal homepage: http://journalofethnicfoods.net Original article Ethnic and traditional Iranian breads: different types, and historical and cultural aspects * Vahid Mohammadpour Karizaki Chemical Engineering Department, Quchan University of Advanced Technology, Quchan, Iran article info abstract Article history: Background: Bread making has a long history in Iran. Because of the inseparable relationship between Received 21 December 2016 Iranian people and bread, an increasingly wide variety of this healthy and nutritious food is prepared and Received in revised form consumed throughout the country. The present work aims at documenting and providing information 14 January 2017 about breads of Iranian cuisine. Accepted 20 January 2017 Methods: The required information was obtained via a direct face-to-face questionnaire-based survey Available online xxx among housewives, domestic people, and Iranian bakers. The statistical society was selected by random sampling among people from the top eight most populous cities in the country. Keywords: bread Results: More than 30 types of ethnic and traditional bread of Iranian cuisine are introduced in two main fi ethnic food categories: the rst group includes breads that are consumed all around the country, and the second Iran group consists of those that are prepared in special regions, or by ethnic groups. Conclusion: The historical and cultural aspects of the Iranian foods showed that bread is the most common and popular food in the country. © 2017 Korea Food Research Institute. Published by Elsevier B.V. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/). -

2020 Annual Recipe SIP.Pdf
SPECIAL COLLECTOR’SEDITION 2020 ANNUAL Every Recipe from a Full Year of America’s Most Trusted Food Magazine CooksIllustrated.com $12.95 U.S. & $14.95 CANADA Cranberry Curd Tart Display until February 22, 2021 2020 ANNUAL 2 Chicken Schnitzel 38 A Smarter Way to Pan-Sear 74 Why and How to Grill Stone 4 Malaysian Chicken Satay Shrimp Fruit 6 All-Purpose Grilled Chicken 40 Fried Calamari 76 Consider Celery Root Breasts 42 How to Make Chana Masala 77 Roasted Carrots, No Oven 7 Poulet au Vinaigre 44 Farro and Broccoli Rabe Required 8 In Defense of Turkey Gratin 78 Braised Red Cabbage Burgers 45 Chinese Stir-Fried Tomatoes 79 Spanish Migas 10 The Best Turkey You’ll and Eggs 80 How to Make Crumpets Ever Eat 46 Everyday Lentil Dal 82 A Fresh Look at Crepes 13 Mastering Beef Wellington 48 Cast Iron Pan Pizza 84 Yeasted Doughnuts 16 The Easiest, Cleanest Way 50 The Silkiest Risotto 87 Lahmajun to Sear Steak 52 Congee 90 Getting Started with 18 Smashed Burgers 54 Coconut Rice Two Ways Sourdough Starter 20 A Case for Grilled Short Ribs 56 Occasion-Worthy Rice 92 Oatmeal Dinner Rolls 22 The Science of Stir-Frying 58 Angel Hair Done Right 94 Homemade Mayo That in a Wok 59 The Fastest Fresh Tomato Keeps 24 Sizzling Vietnamese Crepes Sauce 96 Brewing the Best Iced Tea 26 The Original Vindaloo 60 Dan Dan Mian 98 Our Favorite Holiday 28 Fixing Glazed Pork Chops 62 No-Fear Artichokes Cookies 30 Lion’s Head Meatballs 64 Hummus, Elevated 101 Pouding Chômeur 32 Moroccan Fish Tagine 66 Real Greek Salad 102 Next-Level Yellow Sheet Cake 34 Broiled Spice-Rubbed 68 Salade Lyonnaise Snapper 104 French Almond–Browned 70 Showstopper Melon Salads 35 Why You Should Butter- Butter Cakes 72 Celebrate Spring with Pea Baste Fish 106 Buttermilk Panna Cotta Salad 36 The World’s Greatest Tuna 108 The Queen of Tarts 73 Don’t Forget Broccoli Sandwich 110 DIY Recipes America’s Test Kitchen has been teaching home cooks how to be successful in the kitchen since 1993. -

Maillard Reaction Products: Occurrence, Mitigation Strategies and Their Physiological Relevance
DOI: 10.14267/phd.2015001 MAILLARD REACTION PRODUCTS: OCCURRENCE, MITIGATION STRATEGIES AND THEIR PHYSIOLOGICAL RELEVANCE Vincenzo Fogliano Doctoral Thesis Corvinus University of Budapest Faculty of Food Science Department of Food Chemistry and Nutrition Budapest, 2014 DOI: 10.14267/phd.2015001 PhD School/Program Name: PhD School of Food Science Field: Food Science Head: Prof. József Felföldi, PhD Corvinus University of Budapest Supervisor: Prof. Dr Livia Simon Sarkadi, DSc Department of Food Chemistry and Nutrition Faculty of Food Science Corvinus University of Budapest The applicant met the requirement of the PhD regulations of the Corvinus University of Budapest and the thesis is accepted for the defence process. ............................................... ............................................... Head of PhD School Supervisor i DOI: 10.14267/phd.2015001 According to the Doctoral Council of Life Sciences of Corvinus University of Budapest on 7th October, 2014, the following committee was designated for the public discussion: Committee: Chair: Prof. Dr József Farkas, MHAS, BCE Members: Prof. Dr Péter Fodor, DSc, BCE Prof. Dr András Salgó, DSc, BME Prof. Dr Éva Gelencsér, CSc, NAIK-ÉKI Dr Gabriella Kiskó, PhD, BCE Opponents: Prof. Dr Anna Halász, DSc, NAIK-ÉKI Prof. Dr Péter Biacs, DSc, BCE Secretary: Dr Gabriella Kiskó, PhD, BCE ii DOI: 10.14267/phd.2015001 TABLE OF CONTENT Page 1. General Introduction 1 1.1 Description of the Maillard Reaction 1 1.2 Relevance in different foods 2 1.3 Why is still necessary to study this reaction -
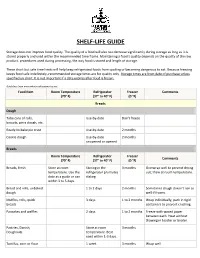
Shelf-Life Guide
SHELF-LIFE GUIDE Storage does not improve food quality. The quality of a food will also not decrease significantly during storage as long as it is stored properly and used within the recommended time frame. Maintaining a food’s quality depends on the quality of the raw product, procedures used during processing, the way food is stored and length of storage. These short but safe time limits will help keep refrigerated foods from spoiling or becoming dangerous to eat. Because freezing keeps food safe indefinitely, recommended storage times are for quality only. Storage times are from date of purchase unless specified on chart. It is not important if a date expires after food is frozen. Guidelines from www.whatscookingamerica.net Food Item Room Temperature Refrigerator Freezer Comments (70° F) (37° to 40° F) (0 °F) Breads Dough Tube cans of rolls, Use-by-date Don't freeze biscuits, pizza dough, etc. Ready-to-bake pie crust Use-by-date 2 months Cookie dough Use-by-date 2 months unopened or opened Breads Room Temperature Refrigerator Freezer Comments (70° F) (37° to 40° F) (0 °F) Breads, fresh Store at room Storing in the 3 months Overwrap well to prevent drying temperature. Use the refrigerator promotes out; thaw at room temperature. date as a guide or use staling. within 3 to 5 days. Bread and rolls, unbaked 1 to 2 days 2 months Sometimes dough doesn't rise as dough well if frozen. Muffins, rolls, quick 3 days 1 to 2 months Wrap individually, pack in rigid breads containers to prevent crushing. -

Sensory and Physicochemical Characterization of Sourdough Bread Prepared with a Coconut Water Kefir Starter
foods Article Sensory and Physicochemical Characterization of Sourdough Bread Prepared with a Coconut Water Kefir Starter Mansi Limbad * , Noemi Gutierrez Maddox, Nazimah Hamid and Kevin Kantono Department of Food Science and Microbiology, Auckland University of Technology, 34, Saint Paul Street, Auckland 1010, New Zealand; [email protected] (N.G.M.); [email protected] (N.H.); [email protected] (K.K.) * Correspondence: [email protected]; Tel.: +64-9-921-9999 (ext. 8384) Received: 18 July 2020; Accepted: 19 August 2020; Published: 24 August 2020 Abstract: There is a recognized need for formulating functional food products using selected lactic acid bacteria (LAB) starter cultures from various sources such as kefir, yoghurt or kombucha that have health benefits. The principle objective of this study was to investigate the use of a coconut water kefir-based fermentation starter culture using Lactobacillus fermentum and Lactobacillus plantarum to develop a sourdough bread. Check-all-that-apply (CATA) sensory profiling was used in this study to evaluate the sensory profile of sourdough breads that varied with culture type, culture concentrations, with and without added yeast, and with fermentation for 18 and 24 h. Based on correspondence analysis (CA) of the CATA results, bread samples with positive sensory attributes were chosen for further physicochemical analysis. Physicochemical analyses (texture, proximate composition, shelf life, carboxylic acid analysis and amino acid analysis) were carried out on breads formulated with starter culture concentrations of 8.30 log CFU/mL of L. fermentum, 4.90 log CFU/mL of L. fermentum and 9.60 log CFU/mL of L. -

Impact of Wort Amino Acids on Beer Flavour: a Review
fermentation Review Impact of Wort Amino Acids on Beer Flavour: A Review Inês M. Ferreira and Luís F. Guido * LAQV/REQUIMTE, Faculdade de Ciências, Universidade do Porto, Rua do Campo Alegre, 687, 4169-007 Porto, Portugal; ines.fi[email protected] * Correspondence: [email protected]; Tel.: +351-220-402-644 Received: 3 March 2018; Accepted: 25 March 2018; Published: 28 March 2018 Abstract: The process by which beer is brewed has not changed significantly since its discovery thousands of years ago. Grain is malted, dried, crushed and mixed with hot water to produce wort. Yeast is added to the sweet, viscous wort, after which fermentation occurs. The biochemical events that occur during fermentation reflect the genotype of the yeast strain used, and its phenotypic expression is influenced by the composition of the wort and the conditions established in the fermenting vessel. Although wort is complex and not completely characterized, its content in amino acids indubitably affects the production of some minor metabolic products of fermentation which contribute to the flavour of beer. These metabolic products include higher alcohols, esters, carbonyls and sulfur-containing compounds. The formation of these products is comprehensively reviewed in this paper. Furthermore, the role of amino acids in the beer flavour, in particular their relationships with flavour active compounds, is discussed in light of recent data. Keywords: amino acids; beer; flavour; higher alcohols; esters; Vicinal Diketones (VDK); sulfur compounds 1. Introduction The process by which beer has been brewed has not changed significantly since its discovery over 2000 years ago. Although industrial equipment is used for modern commercial brewing, the principles are the same. -

Starch Chemistry Staling Causes and Effects
VOLUME 3 /NUMBER 9 Bread Staling Practical technology from Lallemand Inc. Starch Staling Causes and Effects Chemistry TALING REFERS T O the undesirable wrapped bread tastes dry because water has D-glucose is the basic building block of changes (other than microbial spoil- migrated from the crumb to the crust and starch. Its chemical model is a hexagon Sage) that take place between the time from the starch to the gluten. made up of one oxygen atom, five carbon bread is baked and consumed. Understand- Crust softening in wrapped bread is atoms (numbered 1 through 5), and two ing the different aspects of staling and the caused by an increase in moisture from about forms ( alpha- and beta- ) depending on its factors that affect them can help bakers 12 to 28 percent. This changes the dry, crisp, structure at position 1: make better decisions about their formu - pleasant texture of fresh crust into the soft, las, ingredients, processes, and packaging. leathery, unpleasant texture of stale crust. 6CH OH 6CH OH 2 2 Flavor losses and changes occur as 5 O 5 O HH H HOHH ASPECTS OF STALING some flavor components diminish faster 1( ) 1 4 OH H α 4 OH H (β) Crumb firming is caused by changes in than others. The taste o f fresh bread is usu - HO OH HO H 3 2 3 2 starch structure. The starch in wheat flour ally a combination of sweet, salty, and H OH H OH is made up of straight and branched chains slightly sour, but with age the sweet and alpha-glucose beta-glucose contained in granules. -

“Give Us This Day Our Daily Bread”
“Give us this day our daily bread” Rosalba Gentile Abstract Bread is a universal and ancient staple that in Italy is regarded as the symbol of both food and national unity. Nonetheless, the great variety of breads dating back to Roman times and still available in the country recalls the political fragmentation of pre-Unification Italy. Furthermore, as it is essentially a cultural object, bread is closely intertwined with the social structure of Italian society whose cultural and dietary shifts, however, have adversely affected bread production and consumption. The latter, moreover, is also linked to the so- called diseases of civilisation that actually constitute public health problems. The political aspects of bread analysed in this paper mainly pertain to the national and European legislation which establishes the characteristics and names of Italian bread, and to the gradual disappearance of artisan bakery that does not seem to attract the younger generations. The relationship between bread and environment, on the other hand, shows how climate change, pesticides, and fertilisers negatively impact on wheat production. Additionally, bread-making technology and the controversial issue relating to GM crops highlight the unavoidable role of biotechnology in the future nutritional scenario. A scenario that is likely to be dominated by healthy foods such as gluten-free, salt- free and functional breads. Keywords: bread, Italy, cultural object, environment, bread-technology. Introduction Bread has been widely recognised as one of the most ancient and basic foods of mankind;1 a daily staple that has significantly helped to form the foundations of civilisation2 and which still lies at the heart of contemporary society.3 This basically allows to view bread as a sort of “prism” that is apt to imbibe, refract, and eventually unify a great variety of cultural events, thus creating a common and coherent language;4 i.e., a ‘sensory language’ permitting a mutual comprehension between 1 Cauvain, S.P., 1998. -

Triggering the Aroma Production Through Chemical Kinetics, Chemical Engineering Transactions, 52, 985-990 DOI:10.3303/CET1652165 986
985 A publication of CHEMICAL ENGINEERING TRANSACTIONS VOL. 52, 2016 The Italian Association of Chemical Engineering Online at www.aidic.it/cet Guest Editors: Petar Sabev Varbanov, Peng-Yen Liew, Jun-Yow Yong, Jiří Jaromír Klemeš, Hon Loong Lam Copyright © 2016, AIDIC Servizi S.r.l., ISBN 978-88-95608-42-6; ISSN 2283-9216 DOI: 10.3303/CET1652165 Bread as a Chemical Reactor: Triggering the Aroma Production through Chemical Kinetics a a b b Davide Papasidero* , Alessandro Giorgi , Elisa Rocchi , Laura Piazza , Sauro Pierucci a, Giulia Bozzanoa, Flavio Manentia aDipartimento di Chimica, Materiali e Ingegneria Chimica “Giulio Natta”, Politecnico di Milano, Piazza Leonardo da Vinci 32, 20133 Milano, Italy b DeFENS, Dept. of Food, Environmental and Nutritional Sciences, Università degli Studi di Milano, Via Mangiagalli 25, 20133 Milano, Italy [email protected] Recent studies address the structural modifications occurring in the thermal processing of food to interact with the development of aroma and taste compounds. Food sensory science is often related to statistical analysis of panel tests and subjective human responses. From a chemical engineering perspective, food can be treated like a chemical reactor. Bread can be one example of “food chemical reactor”, which enormously changes its density (from high to low depending on the leavening process), its aroma (driven from Maillard and other reactions involving carbohydrates, proteins and fats), its color (caramelization and Maillard), its structure (starch gelatinization, gluten hardening), etc. Indeed, transport phenomena and chemical kinetics are consistently involved in this process. A detailed study on the chemical kinetics of bread aroma development can be helpful to achieve an objective quality marker, which can be monitored and controlled dynamically through the process conditions and variables.