Summary of Product Characteristics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Appendix a Common Abbreviations Used in Medication
UNIVERSITY OF AMSTERDAM MASTERS THESIS Impact of Medication Grouping on Fall Risk Prediction in Elders: A Retrospective Analysis of MIMIC-III Critical Care Database Student: SRP Mentor: Noman Dormosh Dr. Martijn C. Schut Student No. 11412682 – SRP Tutor: Prof. dr. Ameen Abu-Hanna SRP Address: Amsterdam University Medical Center - Location AMC Department Medical Informatics Meibergdreef 9, 1105 AZ Amsterdam Practice teaching period: November 2018 - June 2019 A thesis submitted in fulfillment of the requirements for the degree of Master of Medical Informatics iii Abstract Background: Falls are the leading cause of injury in elderly patients. Risk factors for falls in- cluding among others history of falls, old age, and female gender. Research studies have also linked certain medications with an increased risk of fall in what is called fall-risk-increasing drugs (FRIDs), such as psychotropics and cardiovascular drugs. However, there is a lack of consistency in the definitions of FRIDs between the studies and many studies did not use any systematic classification for medications. Objective: The aim of this study was to investigate the effect of grouping medications at different levels of granularity of a medication classification system on the performance of fall risk prediction models. Methods: This is a retrospective analysis of the MIMIC-III cohort database. We created seven prediction models including demographic, comorbidity and medication variables. Medica- tions were grouped using the anatomical therapeutic chemical classification system (ATC) starting from the most specific scope of medications and moving up to the more generic groups: one model used individual medications (ATC level 5), four models used medication grouping at levels one, two, three and four of the ATC and one model did not include med- ications. -

Summary of Product Characteristics
Summary of Product Characteristics 1. NAME OF THE MEDICINAL PRODUCT MAXITROL ophthalmic ointment 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Ophthalmic ointment: 1 g ointment contains 1 mg dexamethasone, 3,500 I.U. neomycin sulphate (as base) and 6,000 I.U.polymyxin B sulphate. For a full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Ophthalmic ointment White to very pale yellow homogeneous translucent ointment 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Maxitrol ophthalmic ointment is indicated for the treatment of eye infections which are responsive to steroids, when an antibiotic is also needed. 4.2 Posology and method of administration Children and Adults (including the Elderly) Apply a small amount (1-1.5 cm) in the conjunctival sac 3 to 4 times daily, or use as a supplement to the eye drops at bedtime. After application of the ointment, look downward for a moment before closing the eyes. Method of administration: For ocular use. To prevent contamination of the tube tip and ointment, care must be taken not to touch the eyelids, surrounding areas, or other surfaces with the tube tip. Keep the tube tightly closed when not in use. 4.3 Contraindications • Hypersensitivity to the active substances or to any of the excipients listed in section 6.1. • Herpes simplex keratitis. • Vaccinia, varicella, and other viral infection of cornea or conjunctiva. • Fungal diseases of ocular structures. • Mycobacterial ocular infections. 4.4 Special warnings and precautions for use - For topical ophthalmic use only. Not for injection or ingestion. - As with all antibacterial preparation prolonged use may lead to overgrowth of non- susceptible bacterial strains or fungi. -

The Effect of Topical Beta-Adrenoceptor Blocking Agents on Pulsatile Ocular Blood Flow
THE EFFECT OF TOPICAL BETA-ADRENOCEPTOR BLOCKING AGENTS ON PULSATILE OCULAR BLOOD FLOW C. D. MORSMAN, M. E. BOSEM, M. LUSKY and R. N. WEINREB San Diego, California SUMMARY factors (e.g. hypertension, diabetes, peripheral Thirty-three ocular hypertensive patients (21 with vascular disease and vasospasm) to the vascular primary open angle glaucoma and 12 glaucoma system suggest that blood flow in the optic nerve suspects) were randomly assigned to receive either head and retina may be altered in glaucoma.4 Of the timolol, levobunolol or betaxolol in one eye. Pulsatile various vascular beds within the posterior segment, ocular blood flow (POBF) was measured before the choroidal circulation is of particular interest since treatment (baseline) and 2 hours after drop adminis it provides the major contribution to the blood flow tration. After 1 week of regular twice-daily dosage, of the optic nerve at the level of the lamina cribrosa.5 POBF was measured again both immediately before Beta-2 adrenergic receptors have been demon and 2 hours after drop instillation. All measurements strated in human optic nerve,6 as well as in choroidal were made by an investigator masked to treatment. and retinal blood vessels.7 Blockade of these POBF increased by 11% (p = 0.09) at week 0 after receptors can cause vasoconstrictionS which could levobunolol administration, and by 22% (p = 0.20) at adversely affect visual function if adequate concen week 1 before drop administration compared with trations of the drug diffused into the posterior baseline. It dropped by 23% and 25% (p = 0.04 and segment of the eye or were absorbed systemically. -

Use of Emulsions for Intra- and Periocular Injection
(19) & (11) EP 1 611 879 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: A61K 9/107 (2006.01) 12.08.2009 Bulletin 2009/33 (21) Application number: 04291684.1 (22) Date of filing: 02.07.2004 (54) Use of emulsions for intra- and periocular injection Verwendung von Emulsionen zur intra- und periocularen Injection Utilisation des émulsions pour injection intra- et périoculaire. (84) Designated Contracting States: (74) Representative: de Mareüil-Villette, Caroline et al AT BE BG CH CY CZ DE DK EE ES FI FR GB GR Cabinet Plasseraud HU IE IT LI LU MC NL PL PT RO SE SI SK TR 52 rue de la Victoire 75440 Paris Cedex 09 (FR) (43) Date of publication of application: 04.01.2006 Bulletin 2006/01 (56) References cited: EP-A- 0 521 799 EP-A- 1 020 194 (73) Proprietors: WO-A-02/09667 WO-A-93/18852 • Novagali Pharma S.A. WO-A-94/05298 WO-A-03/053405 91000 Evry (FR) US-A- 5 632 984 • CENTRE NATIONAL DE LA RECHERCHE SCIENTIFIQUE (CNRS) • KLANG S H ET AL: "PHYSICOCHEMICAL 75016 Paris (FR) CHARACTERIAZATION AND ACUTE TOXICITY • INSTITUT NATIONAL DE LA SANTE ET DE LA EVALUATION OF A POSITIVELY-CHARGED RECHERCHE MEDICALE (INSERM) SUBMICRON EMULSION VEHICLE" JOURNAL 75013 Paris (FR) OF PHARMACY AND PHARMACOLOGY, • YISSUM RESEARCH DEVELOPMENT COMPANY LONDON, GB, vol. 46, no. 12, December 1994 OF THE HEBREW UNIVERSITY OF JERUSALEM (1994-12), pages 986-993, XP008005426 ISSN: 91390 Jerusalem (IL) 0022-3573 • KLANG S ET AL: "INFLUENCE OF EMULSION (72) Inventors: DROPLET SURFACE CHARGE ON • Rabinovich-Guilatt, Laura INDOMETHACIN OCULAR TISSUE 75015 Paris (FR) DISTRIBUTION" PHARMACEUTICAL • De Kozak, Yvonne DEVELOPMENT AND TECHNOLOGY, NEW 75013 Paris (FR) YORK, NY, US, vol. -

Diabetes, Glycemic Control and Risk of Medical Glaucoma Treatment: a Population-Based Case-Control Study
Clinical Epidemiology Dovepress open access to scientific and medical research Open Access Full Text Article O RIGIN al re S earc H Diabetes, glycemic control and risk of medical glaucoma treatment: A population-based case-control study Lotte G Welinder1 Purpose: To examine the association between diabetes and risk of medical glaucoma treatment Anders H Riis2 and to assess the role of long-term glycemic control in the putative association. Lars L Knudsen1 Design: Population-based case-control study. Reimar W Thomsen2 Methods: Cases of treated glaucoma were all persons filling at least three prescriptions for glaucoma medication for the first time within one year between 2001 and 2006 in Northern 1Department of Ophthalmology, Jutland, Denmark. We used risk set sampling to select 10 gender- and age-matched general 2Department of Clinical Epidemiology, Aalborg Hospital, Aarhus University population controls per case using the Danish Civil Registration System. Data on diabetes, Hospital, Aalborg, Denmark comorbidities, and laboratory tests, including glycosylated hemoglobin (as a measure of glycemic control) were obtained from population-based medical registries. We calculated odds ratio For personal use only. (OR) as an estimate of relative risk for treated glaucoma comparing patients with and without diabetes, adjusted for comorbid conditions and medication use. Results: We included 5,991 persons with incident medical glaucoma treatment and 59,910 population controls. The adjusted OR for treated glaucoma for patients with diabetes was 1.81 (95% confidence interval: 1.65–1.98). The strength of the association between diabetes and glaucoma risk did not vary by diabetes duration or by the level of glycemic control. -
Guidance for the Format and Content of the Protocol of Non-Interventional
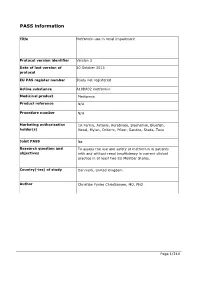
PASS information Title Metformin use in renal impairment Protocol version identifier Version 2 Date of last version of 30 October 2013 protocol EU PAS register number Study not registered Active substance A10BA02 metformin Medicinal product Metformin Product reference N/A Procedure number N/A Marketing authorisation 1A Farma, Actavis, Aurobindo, Biochemie, Bluefish, holder(s) Hexal, Mylan, Orifarm, Pfizer, Sandoz, Stada, Teva Joint PASS No Research question and To assess the use and safety of metformin in patients objectives with and without renal insufficiency in current clinical practice in at least two EU Member States. Country(-ies) of study Denmark, United Kingdom Author Christian Fynbo Christiansen, MD, PhD Page 1/214 Marketing authorisation holder(s) Marketing authorisation N/A holder(s) MAH contact person N/A Page 2/214 1. Table of Contents PASS information .......................................................................................................... 1 Marketing authorisation holder(s) .................................................................................... 2 1. Table of Contents ...................................................................................................... 3 2. List of abbreviations ................................................................................................... 4 3. Responsible parties .................................................................................................... 5 4. Abstract .................................................................................................................. -

Summary of Product Characteristics
Health Products Regulatory Authority Summary of Product Characteristics 1 NAME OF THE MEDICINAL PRODUCT Latanoprost 50 micrograms/ml Eye Drops, Solution 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml of solution contains 50 micrograms of latanoprost. One drop of the solution contains approximately 1.5 micrograms of latanoprost. Excipients with known effect Each ml of solution contains 0.2 mg benzalkonium chloride and 6.34 mg phosphate. For the full list of excipients, see section 6.1. 3 PHARMACEUTICAL FORM Eye drops, solution A clear, colourless, aqueous solution, pH between 6.5 – 6.9 and osmolality between 240 – 294 mOsmol/kg. 4 CLINICAL PARTICULARS 4.1 Therapeutic Indications Reduction of elevated intraocular pressure in patients with open angle glaucoma and ocular hypertension. Reduction of elevated intraocular pressure in paediatric patients with elevated intraocular pressure and paediatric glaucoma. 4.2 Posology and method of administration Posology Adults (including the elderly): Recommended therapy is one eye drop in the affected eye(s) once daily. Optimal effect is obtained if Latanoprost is administered in the evening. The dosage of Latanoprost should not exceed once daily since it has been shown that more frequent administration decreases the intraocular pressure lowering effect. If one dose is missed, treatment should continue with the next dose as normal. Paediatric population: Latanoprost eye drops may be used in paediatric patients at the same posology as in adults. No data are available for preterm infants (less than 36 weeks gestational age). Data in the age group < 1 year (4 patients) are very limited (see section 5.1). Method of administration ______________________________________________________________________________________________________________________ Date Printed 14/08/2018 CRN 2210576 page number: 1 Health Products Regulatory Authority For ocular use. -

Canine Red Eye Elizabeth Barfield Laminack, DVM; Kathern Myrna, DVM, MS; and Phillip Anthony Moore, DVM, Diplomate ACVO
PEER REVIEWED Clinical Approach to the CANINE RED EYE Elizabeth Barfield Laminack, DVM; Kathern Myrna, DVM, MS; and Phillip Anthony Moore, DVM, Diplomate ACVO he acute red eye is a common clinical challenge for tion of the deep episcleral vessels, and is characterized general practitioners. Redness is the hallmark of by straight and immobile episcleral vessels, which run Tocular inflammation; it is a nonspecific sign related 90° to the limbus. Episcleral injection is an external to a number of underlying diseases and degree of redness sign of intraocular disease, such as anterior uveitis and may not reflect the severity of the ocular problem. glaucoma (Figures 3 and 4). Occasionally, episcleral Proper evaluation of the red eye depends on effective injection may occur in diseases of the sclera, such as and efficient diagnosis of the underlying ocular disease in episcleritis or scleritis.1 order to save the eye’s vision and the eye itself.1,2 • Corneal Neovascularization » Superficial: Long, branching corneal vessels; may be SOURCE OF REDNESS seen with superficial ulcerative (Figure 5) or nonul- The conjunctiva has small, fine, tortuous and movable vessels cerative keratitis (Figure 6) that help distinguish conjunctival inflammation from deeper » Focal deep: Straight, nonbranching corneal vessels; inflammation (see Ocular Redness algorithm, page 16). indicates a deep corneal keratitis • Conjunctival hyperemia presents with redness and » 360° deep: Corneal vessels in a 360° pattern around congestion of the conjunctival blood vessels, making the limbus; should arouse concern that glaucoma or them appear more prominent, and is associated with uveitis (Figure 4) is present1,2 extraocular disease, such as conjunctivitis (Figure 1). -

WO 2014/066775 Al 1 May 2014 (01.05.2014) W P O PCT
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2014/066775 Al 1 May 2014 (01.05.2014) W P O PCT (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every A61F 9/00 (2006.01) kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, (21) International Application Number: BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, PCT/US20 13/066834 DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (22) International Filing Date: HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, 25 October 2013 (25.10.201 3) KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, (25) Filing Language: English OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, (26) Publication Language: English SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, (30) Priority Data: ZW. 61/719,144 26 October 2012 (26. 10.2012) US (84) Designated States (unless otherwise indicated, for every (71) Applicant: FORSIGHT VISION5, INC. [US/US]; 191 kind of regional protection available): ARIPO (BW, GH, Jefferson Drive, Menlo Park, CA 94025 (US). GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, SZ, TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, TJ, (72) Inventors: RUBIN, Anne, Brody; 191 Jefferson Drive, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, DK, Menlo Park, CA 94025 (US). -

Antiglaucoma Compositions Containing Combinations of Alpha-2 Agonists and Beta-Blockers
~" ' MM II II II MM II II II Ml II II I II J European Patent Office _ _ _ © Publication number: 0 365 662 B1 Office europeen* des.. brevets , © EUROPEAN PATENT SPECIFICATION © Date of publication of patent specification: 09.03.94 © Int. CI.5: A61 K 31/47 © Application number: 89905874.7 @ Date of filing: 26.04.89 © International application number: PCT/US89/01994 © International publication number: WO 89/10126 (02.11.89 89/26) (54) ANTIGLAUCOMA COMPOSITIONS CONTAINING COMBINATIONS OF ALPHA-2 AGONISTS AND BETA-BLOCKERS. ® Priority: 26.04.88 US 186504 1075-1078 @ Date of publication of application: J. of Cardiovascular Pharmacology, vol. 2, 02.05.90 Bulletin 90/18 suppl. 1, 1980, S. 21-28 © Publication of the grant of the patent: © Proprietor: ALCON LABORATORIES INC 09.03.94 Bulletin 94/10 6201 South Freeway Ft. Worth, TX 76134(US) © Designated Contracting States: AT BE CH DE FR GB IT LI LU NL SE @ Inventor: DE SANTIS, Louis, M. 2316 Wlnton Terrace West © References cited: Fort Worth, TX 761 09(US) US-A- 4 455 317 US-A- 4 515 800 © Representative: Jump, Timothy John Simon et AM A DRUG EVALUATION, 2nd ed., 1973; al "Agent used to treat Glaucoma", pp. 675-686. Venner Shipley & Co. 20 Little Britain GLAUCOMA, vol. 1, no. 1, February 1979; London EC1A 7DH (GB) 00 S.SUGAR, pp. 9-15. CM CO Ophthalmology, vol. 96, no. 1, 1989, p. 3-7 CO m Ophthalmology, vol. 98, no. 7, 1991, p. CO 00 Note: Within nine months from the publication of the mention of the grant of the European patent, any person may give notice to the European Patent Office of opposition to the European patent granted. -

Supplementary File Table S1. List of Glucocorticoids and Corresponding
Supplementary file Table S1. List of glucocorticoids and corresponding identification codes Table S2. List of concurrent drugs reimbursed at index date and corresponding identification codes Table S3. List of comorbidities at risk for glucocorticoid users and corresponding identification codes Table S4. List of recognized indications of glucocorticoid therapy and corresponding identification codes Table S5. List of therapeutic measures associated with the prescription of glucocorticoids and corresponding identification codes Figure S1. Trends in prevalence of oral glucocorticoid use in France per year from 2007 to 2014 by products. A) Prevalence estimates with 95%CI (error bars) and B) relative changes in reference to year 2007 Figure S2. Trends in prevalence of oral glucocorticoid (GC) use in France per year from 2007 to 2014, in women (A) and men (B) according to age. Prevalence estimates with 95% CIs (error bars) 1 Table S1. List of glucocorticoids and corresponding identification codes Glucocorticoids Source Code Betamethasone Drug reimbursement (ATC) H02AB01 Dexamethasone Drug reimbursement (ATC) H02AB02 Methylprednisolone Drug reimbursement (ATC) H02AB04 Prednisolone Drug reimbursement (ATC) H02AB06 Prednisone Drug reimbursement (ATC) H02AB07 ATC: Anatomical Therapeutic Chemical classification system Table S2. List of concurrent drugs reimbursed at index date and corresponding identification codes Concurrent drugs at index date Source Code Analgesics Drug reimbursement (ATC) N02 Antibiotics Drug reimbursement (ATC) J01 Anti-inflammatory -

Glaucoma Medical Treatment: Philosophy, Principles and Practice
Glaucoma medical CLIVE MIGDAL treatment: philosophy, principles and practice Abstract assessment of these parameters. Indeed There have been numerous recent advances in compounds are under evaluation that affect the the management of glaucoma, not least the function of the optic nerve (via improved blood development of new drugs to help manage supply or improved neuronal cell physiology) raised intraocular pressure. In addition, the but may or may not lower lOP. It may even be concepts of improving blood flow to the optic possible in the future to therapeutically alter the nerve head and neuroprotection are currently human genome, genetically deliver provoking considerable interest. This article neuroprotective substances or aid regeneration considers the aims and philosophy of of the optic nerve axons. glaucoma drug therapy, summarises some of The main aim of glaucoma therapy must still the basic facts and principles of modem be the preservation of visual function. At the glaucoma medications, and suggests a same time, the therapy should not have adverse practical approach to the choice of therapy. side effects and should not affect the quality of life of the patient (by causing either side effects Key words Blood flow, Intraocular pressure, or inconvenience and disruption of daily Neuroprotection, Primary open angle glaucoma, Topical medications lifestyle). The cost of the therapy, both direct and indirect, must also be taken into consideration.s Currently, typical glaucoma management Philosophy consists of lowering the lOP to a satisfactory Primary open-angle glaucoma is a complex and safe target leve1.6 To determine the success disease for which a number of risk factors have of this treatment, the patient must be followed been identified, including intraocular pressure, long-term with routine assessment of lOP, discs age, race and family history.l,2 Due to our and fields to exclude progressive damage.