Substance Activity
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Clinical Study Is Nonmicronized Diosmin 600Mg As Effective As
Hindawi International Journal of Vascular Medicine Volume 2020, Article ID 4237204, 9 pages https://doi.org/10.1155/2020/4237204 Clinical Study Is Nonmicronized Diosmin 600mg as Effective as Micronized Diosmin 900mg plus Hesperidin 100mg on Chronic Venous Disease Symptoms? Results of a Noninferiority Study Marcio Steinbruch,1 Carlos Nunes,2 Romualdo Gama,3 Renato Kaufman,4 Gustavo Gama,5 Mendel Suchmacher Neto,6 Rafael Nigri,7 Natasha Cytrynbaum,8 Lisa Brauer Oliveira,9 Isabelle Bertaina,10 François Verrière,10 and Mauro Geller 3,6,9 1Hospital Albert Einstein (São Paulo-Brasil), R. Mauricio F Klabin 357/17, Vila Mariana, SP, Brazil 04120-020 2Instituto de Pós-Graduação Médica Carlos Chagas-Fundação Educacional Serra dos Órgãos-UNIFESO (Rio de Janeiro/Teresópolis- Brasil), Av. Alberto Torres 111, Teresópolis, RJ, Brazil 25964-004 3Fundação Educacional Serra dos Órgãos-UNIFESO (Teresópolis-Brasil), Av. Alberto Torres 111, Teresópolis, RJ, Brazil 25964-004 4Faculdade de Ciências Médicas, Universidade Estadual do Rio de Janeiro (UERJ) (Rio de Janeiro-Brazil), Av. N. Sra. De Copacapana, 664/206, Rio de Janeiro, RJ, Brazil 22050-903 5Fundação Educacional Serra dos Órgãos-UNIFESO (Teresópolis-Brasil), Rua Prefeito Sebastião Teixeira 400/504-1, Rio de Janeiro, RJ, Brazil 25953-200 6Instituto de Pós-Graduação Médica Carlos Chagas (Rio de Janeiro-Brazil), R. General Canabarro 68/902, Rio de Janeiro, RJ, Brazil 20271-200 7Department of Medicine, Rutgers New Jersey Medical School-USA, 185 S Orange Ave., Newark, NJ 07103, USA 8Hospital Universitário Pedro Ernesto, Universidade Estadual do Rio de Janeiro (UERJ) (Rio de Janeiro-Brazil), R. Hilário de Gouveia, 87/801, Rio de Janeiro, RJ, Brazil 22040-020 9Universidade Federal do Rio de Janeiro (UFRJ) (Rio de Janeiro-Brazil), Av. -

Properties and Units in Clinical Pharmacology and Toxicology
Pure Appl. Chem., Vol. 72, No. 3, pp. 479–552, 2000. © 2000 IUPAC INTERNATIONAL FEDERATION OF CLINICAL CHEMISTRY AND LABORATORY MEDICINE SCIENTIFIC DIVISION COMMITTEE ON NOMENCLATURE, PROPERTIES, AND UNITS (C-NPU)# and INTERNATIONAL UNION OF PURE AND APPLIED CHEMISTRY CHEMISTRY AND HUMAN HEALTH DIVISION CLINICAL CHEMISTRY SECTION COMMISSION ON NOMENCLATURE, PROPERTIES, AND UNITS (C-NPU)§ PROPERTIES AND UNITS IN THE CLINICAL LABORATORY SCIENCES PART XII. PROPERTIES AND UNITS IN CLINICAL PHARMACOLOGY AND TOXICOLOGY (Technical Report) (IFCC–IUPAC 1999) Prepared for publication by HENRIK OLESEN1, DAVID COWAN2, RAFAEL DE LA TORRE3 , IVAN BRUUNSHUUS1, MORTEN ROHDE1, and DESMOND KENNY4 1Office of Laboratory Informatics, Copenhagen University Hospital (Rigshospitalet), Copenhagen, Denmark; 2Drug Control Centre, London University, King’s College, London, UK; 3IMIM, Dr. Aiguader 80, Barcelona, Spain; 4Dept. of Clinical Biochemistry, Our Lady’s Hospital for Sick Children, Crumlin, Dublin 12, Ireland #§The combined Memberships of the Committee and the Commission (C-NPU) during the preparation of this report (1994–1996) were as follows: Chairman: H. Olesen (Denmark, 1989–1995); D. Kenny (Ireland, 1996); Members: X. Fuentes-Arderiu (Spain, 1991–1997); J. G. Hill (Canada, 1987–1997); D. Kenny (Ireland, 1994–1997); H. Olesen (Denmark, 1985–1995); P. L. Storring (UK, 1989–1995); P. Soares de Araujo (Brazil, 1994–1997); R. Dybkær (Denmark, 1996–1997); C. McDonald (USA, 1996–1997). Please forward comments to: H. Olesen, Office of Laboratory Informatics 76-6-1, Copenhagen University Hospital (Rigshospitalet), 9 Blegdamsvej, DK-2100 Copenhagen, Denmark. E-mail: [email protected] Republication or reproduction of this report or its storage and/or dissemination by electronic means is permitted without the need for formal IUPAC permission on condition that an acknowledgment, with full reference to the source, along with use of the copyright symbol ©, the name IUPAC, and the year of publication, are prominently visible. -

)&F1y3x PHARMACEUTICAL APPENDIX to THE
)&f1y3X PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE )&f1y3X PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 3 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. Product CAS No. Product CAS No. ABAMECTIN 65195-55-3 ACTODIGIN 36983-69-4 ABANOQUIL 90402-40-7 ADAFENOXATE 82168-26-1 ABCIXIMAB 143653-53-6 ADAMEXINE 54785-02-3 ABECARNIL 111841-85-1 ADAPALENE 106685-40-9 ABITESARTAN 137882-98-5 ADAPROLOL 101479-70-3 ABLUKAST 96566-25-5 ADATANSERIN 127266-56-2 ABUNIDAZOLE 91017-58-2 ADEFOVIR 106941-25-7 ACADESINE 2627-69-2 ADELMIDROL 1675-66-7 ACAMPROSATE 77337-76-9 ADEMETIONINE 17176-17-9 ACAPRAZINE 55485-20-6 ADENOSINE PHOSPHATE 61-19-8 ACARBOSE 56180-94-0 ADIBENDAN 100510-33-6 ACEBROCHOL 514-50-1 ADICILLIN 525-94-0 ACEBURIC ACID 26976-72-7 ADIMOLOL 78459-19-5 ACEBUTOLOL 37517-30-9 ADINAZOLAM 37115-32-5 ACECAINIDE 32795-44-1 ADIPHENINE 64-95-9 ACECARBROMAL 77-66-7 ADIPIODONE 606-17-7 ACECLIDINE 827-61-2 ADITEREN 56066-19-4 ACECLOFENAC 89796-99-6 ADITOPRIM 56066-63-8 ACEDAPSONE 77-46-3 ADOSOPINE 88124-26-9 ACEDIASULFONE SODIUM 127-60-6 ADOZELESIN 110314-48-2 ACEDOBEN 556-08-1 ADRAFINIL 63547-13-7 ACEFLURANOL 80595-73-9 ADRENALONE -

Title 16. Crimes and Offenses Chapter 13. Controlled Substances Article 1
TITLE 16. CRIMES AND OFFENSES CHAPTER 13. CONTROLLED SUBSTANCES ARTICLE 1. GENERAL PROVISIONS § 16-13-1. Drug related objects (a) As used in this Code section, the term: (1) "Controlled substance" shall have the same meaning as defined in Article 2 of this chapter, relating to controlled substances. For the purposes of this Code section, the term "controlled substance" shall include marijuana as defined by paragraph (16) of Code Section 16-13-21. (2) "Dangerous drug" shall have the same meaning as defined in Article 3 of this chapter, relating to dangerous drugs. (3) "Drug related object" means any machine, instrument, tool, equipment, contrivance, or device which an average person would reasonably conclude is intended to be used for one or more of the following purposes: (A) To introduce into the human body any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (B) To enhance the effect on the human body of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (C) To conceal any quantity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; or (D) To test the strength, effectiveness, or purity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state. (4) "Knowingly" means having general knowledge that a machine, instrument, tool, item of equipment, contrivance, or device is a drug related object or having reasonable grounds to believe that any such object is or may, to an average person, appear to be a drug related object. -

Calcium Dobesilate Versus Micronised Purified Flavonoid Fraction of Diosmin in the Treatment of Chronic Venous Disease: a Randomized Prospective Study
Acta Medica Mediterranea, 2018, 34: 657 CALCIUM DOBESILATE VERSUS MICRONISED PURIFIED FLAVONOID FRACTION OF DIOSMIN IN THE TREATMENT OF CHRONIC VENOUS DISEASE: A RANDOMIZED PROSPECTIVE STUDY EMINE SEYMA DENLI YALVAC1, MURAT DEMIROĞLU2, SIDIKA GURSEL1, EBUZER AYDIN1 ¹Medeniyet University Faculty of Medicine, Department of Cardiovascular Surgery, Istanbul, Turkey - 2Medeniyet University Faculty of Medicine, Department of Department of Orthopedics and Traumatology, Istanbul, Turkey ABSTRACT Introduction: Chronic venous disease (CVD) of the lower extremities has a substantial effect on quality of life. The clinical, etiology, anatomy, pathophysiology classification (CEAP) is a frequently used classification for CVD. Patients with varicose veins are classified as CEAP class C2. Veno-active drugs (VAD) such as Calcium Dobesilate or Micronised Purified Flavonoid Fraction of Diosmin (MPFF) reduce the symptoms of pain associated with CVD. However, although the effectiveness of VAD is well established, it is controversial. The aims of the this study were to compare the efficacy of two VAD (Calcium dobesilate and MPFF in the treat- ment of CVD in view of the intended visual analogue scale (VAS) and duplex scanning (DS) changes such as measuring great saphe- nous vein (GSV) diameter. Materials and methods: A total of 24 patients who had no prior VAD treatment with CEAP class C2, with no family history of CVD were treated; 12 received calcium dobesilate and 12 received MPFF for 60 days. Comorbidities such as hypertension (HT) and diabetes mellitus (DM) were examined. Before and after treatment period, the patients were investigated with DS for diameter of GSV and pain complaint according to VAS were recorded. Results: There were no difference in treatment groups in terms of age, gender, or the comorbidities. -

Appendices Feb 2010 Inclusion of Cobalt and 2019 Fei Prohibited
APPENDIX 2 CLASSIFICATION OF PROHIBITED SUBSTANCES 1. This classification describes the types of Prohibited Substances and places same in specific categories. The list shall be published on the Club’s Notice Board. CLASS I Drugs that have the greatest pharmacological potential to affect the racing performance of a horse and which have no accepted medical use in a racehorse. These include substances which are potent stimulants of the Central Nervous System (CNS). Examples of drugs in this Class include, but are not limited to: Amphetamines, fentanyl, etorphine, cocaine, morphine, meperidine, opiates, opium derivatives, synthetic opiods, psychoactive drugs. CLASS II Drugs that have a high pharmacological potential for affecting the racing performance of a horse. These include: (i) substances which are pharmacologically active in altering the cons (ii) substances which are not generally accepted as therapeutic agents in the racing horse; and (iii) substances, some of which may have legitimate use in equine medicine but should not be found in the racing horse, such as injectable local anaesthetics. 1 Groups of drugs in this Class include, but are not limited to: (a) Opiate partial agonists, or agonistsantagonists; (b) Non-opiate psychotropic drugs, which may have stimulant, depressant, analgesic or neuroleptic effects; (c) Miscellaneous drugs which might have a stimulant effect on the CNS; (d) Drugs with prominent CNS depressant action; (e) Antidepressant and antipsychotic drugs, with or without prominent CNS stimulatory or depressant effects; (f) Muscle blocking drugs which have direct neuromuscular blocking action; (g) Local anaesthetics which have a reasonable potential for use as nerve blocking agents (except procaine); and (h) Snake venom and other biologic substances which may be used as nerve blocking agents. -

Partial Agreement in the Social and Public Health Field
COUNCIL OF EUROPE COMMITTEE OF MINISTERS (PARTIAL AGREEMENT IN THE SOCIAL AND PUBLIC HEALTH FIELD) RESOLUTION AP (88) 2 ON THE CLASSIFICATION OF MEDICINES WHICH ARE OBTAINABLE ONLY ON MEDICAL PRESCRIPTION (Adopted by the Committee of Ministers on 22 September 1988 at the 419th meeting of the Ministers' Deputies, and superseding Resolution AP (82) 2) AND APPENDIX I Alphabetical list of medicines adopted by the Public Health Committee (Partial Agreement) updated to 1 July 1988 APPENDIX II Pharmaco-therapeutic classification of medicines appearing in the alphabetical list in Appendix I updated to 1 July 1988 RESOLUTION AP (88) 2 ON THE CLASSIFICATION OF MEDICINES WHICH ARE OBTAINABLE ONLY ON MEDICAL PRESCRIPTION (superseding Resolution AP (82) 2) (Adopted by the Committee of Ministers on 22 September 1988 at the 419th meeting of the Ministers' Deputies) The Representatives on the Committee of Ministers of Belgium, France, the Federal Republic of Germany, Italy, Luxembourg, the Netherlands and the United Kingdom of Great Britain and Northern Ireland, these states being parties to the Partial Agreement in the social and public health field, and the Representatives of Austria, Denmark, Ireland, Spain and Switzerland, states which have participated in the public health activities carried out within the above-mentioned Partial Agreement since 1 October 1974, 2 April 1968, 23 September 1969, 21 April 1988 and 5 May 1964, respectively, Considering that the aim of the Council of Europe is to achieve greater unity between its members and that this -

Rabe E, Ballarini S, Lehr L; Doxium EDX09/01 Study Group
1: Rabe E, Ballarini S, Lehr L; Doxium EDX09/01 Study Group. A randomized, double-blind, placebo-controlled, clinical study on the efficacy and safety of calcium dobesilate in the treatment of chronic venous insufficiency. Phlebology. 2015 May 18. pii: 0268355515586097. [Epub ahead of print] PubMed PMID: 25991692. 2: Rabe E, Agus GB, Roztocil K. Analysis of the effects of micronised purified flavonoid fraction versus placebo on symptoms and quality of life in patients suffering from chronic venous disease: from a prospective randomised trial. Int Angiol. 2015 May 14. [Epub ahead of print] PubMed PMID: 25972136. 3: Mosti G, De Maeseneer M, Cavezzi A, Parsi K, Morrison N, Nelzen O, Rabe E, Partsch H, Caggiati A, Simka M, Obermayer A, Malouf M, Flour M, Maleti O, Perrin M, Reina L, Kalodiki E, Mannello F, Rerkasem K, Cornu-Thenard A, Chi YW, Soloviy M, Bottini O, Mendyk N, Tessari L, Varghese R, Etcheverry R, Pannier F, Lugli M, Carvallo Lantz AJ, Zamboni P, Zuolo M, Godoy MF, Godoy JM, Link DP, Junger M, Scuderi A. Society for Vascular Surgery and American Venous Forum Guidelines on the management of venous leg ulcers: the point of view of the International Union of Phlebology. Int Angiol. 2015 Jun;34(3):202-18. Epub 2015 Apr 21. PubMed PMID: 25896614. 4: Pannier F, Rabe E. Progression in venous pathology. Phlebology. 2015 Mar;30(1 Suppl):95-7. doi: 10.1177/0268355514568847. Review. PubMed PMID: 25729075. 5: Rabe E, Pannier F. Embolization is not essential in the treatment of leg varices due to pelvic venous insufficiency. -
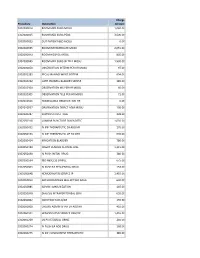
Procedure Description Charge Amount 0302000014 ROOM MED
Charge Procedure Description Amount 0302000014 ROOM MED SURG MOSU 1,040.00 0302000015 ROOM MED SURG PEDS 3,500.00 0302000033 OUT PATIENT BED MOSU 0.00 0302000035 ROOM INTERMEDIATE MOSU 2,055.00 0302000043 ROOM HOSPICE MOSU 805.00 0302000045 ROOM MED SURG W TELE MOSU 1,530.00 0302000050 OBSERVATION INTERM PER HR MOSU 97.00 0302002283 FECAL MANAGEMENT SYSTEM 694.00 0302010232 CATH INDWELL BLADDER SIMPLE 185.00 0302010304 OBSERVATION MS PER HR MOSU 60.00 0302010305 OBSERVATION TELE PER HR MOSU 71.00 0302010556 NONBILLABLE OBSERVATION HR 0.00 0302010557 OBSERVATION DIRECT ADM MOSU 130.00 0302020287 SUPPLIES CHEST TUBE 228.00 0302050318 LUMBAR PUNCTURE DIAGNOSTIC 1,035.00 0302050451 IV INF THERAPEUTIC EA ADD HR 170.00 0302050453 IV INF THERAPEUTIC UP TO 1HR 635.00 0302050454 IRRIGATION BLADDER 780.00 0302050480 INSERT VENOUS CENTRAL LINE 1,315.00 0302050490 IV PUSH INITIAL DRUG 380.00 0302050534 I&D ABSCESS SIMPLE 675.00 0302050603 IV PUSH EA SEQUENTIAL DRUG 153.00 0302050648 HEMODIALYSIS SERVICE IP 1,455.00 0302050813 ARTHROCENTESIS MAJ JNT WO IMAG 630.00 0302050885 ADMIN IMMUNIZATION 145.00 0302050948 DIALYSIS INTRAPERITONEAL SERV 655.00 0302060002 INJECTION SUB-Q/IM 155.00 0302060008 CHEMO ADMIN IV INF EA ADD HR 410.00 0302060101 HEMODIALYSIS SERVICE OBS/OP 1,455.00 0302060269 US PV RESIDUAL URINE 240.00 0302060274 IV PUSH EA ADD DRUG 168.00 0302060275 IV INF CONCURRENT THERAPEUTIC 385.00 0302060276 IV INF SEQUENTIAL THER UP TO 1 191.00 0302060293 INSERT STRAIGHT CATH THERAPEUT 185.00 0302060372 CHEMO ADMIN IV INF SEQ 1 HR 525.00 0302060373 -
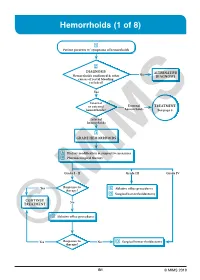
Hemorrhoids (1 of 8)
Hemorrhoids (1 of 8) 1 Patient presents w/ symptoms of hemorrhoids 2 DIAGNOSIS No ALTERNATIVE Hemorrhoids confi rmed & other DIAGNOSIS causes of rectal bleeding excluded? Yes Internal or external External TREATMENT hemorrhoids? hemorrhoids See page 3 Internal hemorrhoids 3 GRADE HEMORRHOIDS A Dietary modifi cation & supportive measures B Pharmacological therapy Grade I - II Grade III Grade IV Yes Response to C Ablative offi ce procedures therapy? D Surgical hemorrhoidectomy CONTINUE No TREATMENT C Ablative offi ce procedures Yes Response to No D Surgical hemorrhoidectomy ©therapy? MIMS B1 © MIMS 2019 Hemorrhoids (2 of 8) 1 SYMPTOMS ATTRIBUTED TO HEMORRHOIDS • Rectal bleeding - Most common presenting symptom - Bright red blood which may drip or squirt into the toilet bowl or scanty amounts may be seen on toilet tissue • Discomfort due to rectal protrusion or lump • Anal pain • HEMORRHOIDS Anal itching 2 DIAGNOSIS Medical History • Assess nature, duration & severity of symptoms - Ask about bleeding, its amount & frequency - Ask about presence of prolapsing tissue, its timing & reproducibility • Elicit possible risk factors for development of hemorrhoidal symptoms - Low-fi ber diets cause small-caliber stools, resulting in straining during defecation & engorgement of hemorrhoids - Prolonged sitting on a toilet which may cause a problem in the venous return in the perianal area - Pregnancy - Advanced age • Th e signs & symptoms of hemorrhoids are not specifi c to the disease, so care must be taken to avoid missing other causes of pathology • Obtain -

PHARMACEUTICAL Antibiotics and Antibacterial
PHARMACEUTICAL Antibiotics and Antibacterial Active Ingredient Form Strength Pack Type Packing Style Amoxicillin Sodium Dry Powder Injections 250mg, 500mg, 1000 mg Vial 1's Amoxicillin Suspension 125mg/5ml, 250mg/5ml Bottle 30ml, 60ml Amoxicillin Dispersible Tablet 125mg, 250mg Strip 10's Amoxicillin Trihydrate Tablet 1000mg Blister 10's Amoxicillin + Clavulanate Potassium Injection 500mg + 100mg, 1g + 200mg Vial 10ml, 20ml Amoxicillin + Clavulanate Potassium Suspension 200mg + 28.5mg Bottle 30ml Amoxicillin + clavulanic Acid Tablets 250mg + 125mg, 500mg + 125mg Strip , Alu-Alu 6's, 10's Amoxicillin + Cloxacillin Capsules 250mg + 250mg, 500mg + 500mg Blister 10's Amoxicillin + Cloxacillin Dry Powder Injections 250mg + 250mg Vial 1's Amoxiciilin + Cloxacillin + Lactic Acid Tablet 250 mg + 250 mg + 60 million Tablet 10's Bacillus Spores, 250 + 250 + 120 million Spores Amoxicillin + Flucloxacillin Capsules 250mg + 250mg Strip 10's Azithromycin Tablets 250mg, 500mg Blister 6's, 3's Azithromycin + Fluconazole + Secnidazole Tablet 1gm + 150mg + 1gm Strip 1 + 1 + 2 Betamethasone Valerate + Neomycin Cream 0.1% + 0.5% Tube 10g Sulphate Beclomethasone + Phenylephrine Hcl + Cream 0.025% + 0.10% + 2.5% + 0.1% Tube 10g Lignocaine Hcl + Chlorocresol Betamethasone Valerate Lotion 0.10% Bottle 30ml Betamethasone Injection 4mg/ml Ampoule 1ml Bethamethasone Opthalmic Solution 0.1% w/v Bottle 5ml Betamethasone Valerate Cream 0.1%w/w Tube 15 g Bethamethasone + Gentamicin Cream 0.1% + 0.05% Tube 10 g Betamethasone Sodium Phosphate Tablet 0.5mg Blister 10s -

Download This Issue
UIP Chapter Meeting - August 25th -27th 2019, Krakow – Poland Contents Foreword 83 UIP Chapter Meeting August 25th -27th 2019, Krakow – Poland 85 Charing Cross International Symposium - Vascular & Endovascular Challenges Update April 15th-18th 2019, London – UK 191 81 Vol 26. No. 3. 2019 Medical Reporters’ Academy The reports from the UIP chapter meeting and Charing cross international symposium were prepared by the following members of the Medical Reporters’ Academy: Roman BREDIKHIN (Russia) Kirill LOBASTOV (Russia) Daniela MASTROIACOVO (Italy) Mustafa SIRLAK (Turkey) Stanislava TZANEVA (Austria) and chaired by: Andrew NICOLAIDES (Cyprus) 82 UIP Chapter Meeting - August 25th -27th 2019, Krakow – Poland Foreword The Medical Reporters’ Academy was co-founded in 1994 as a joint initiative by clinical and research venous disease specialists and Servier; with the main objective to develop an international group of young specialists with a core interest in venous disease drawn from various fields including dermatology, vascular surgery, angiology and/or phlebology. Each year the Medical Reporters’ Academy members are invited by Servier, to cover one of the most important international congresses for venous disease specialists. This year, the UIP Chapter Meeting, which was held in Krakow, Poland on August 25th- 27th, 2019 and the Charing Cross International Symposium: Vascular & Endovascular Challenges Update which was held in London, United Kingdom on April 15th - 18th, 2019 were selected because these congresses involved renowned international and national venous experts and young health care professionals providing a great opportunity to exchange ideas, explore strategies for vein care and discuss the latest trends & innovations in the field. Together with Andrew Nicolaides, the chairman of the group, the academy members explored the program of the congress, selected the events and presentations likely to present breakthroughs or new findings to attend, and wrote short reports.