DAT Organic Chemistry Reaction Summary Sheet Alkene Reactions Hydrohalogenation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Prebiological Evolution and the Metabolic Origins of Life
Prebiological Evolution and the Andrew J. Pratt* Metabolic Origins of Life University of Canterbury Keywords Abiogenesis, origin of life, metabolism, hydrothermal, iron Abstract The chemoton model of cells posits three subsystems: metabolism, compartmentalization, and information. A specific model for the prebiological evolution of a reproducing system with rudimentary versions of these three interdependent subsystems is presented. This is based on the initial emergence and reproduction of autocatalytic networks in hydrothermal microcompartments containing iron sulfide. The driving force for life was catalysis of the dissipation of the intrinsic redox gradient of the planet. The codependence of life on iron and phosphate provides chemical constraints on the ordering of prebiological evolution. The initial protometabolism was based on positive feedback loops associated with in situ carbon fixation in which the initial protometabolites modified the catalytic capacity and mobility of metal-based catalysts, especially iron-sulfur centers. A number of selection mechanisms, including catalytic efficiency and specificity, hydrolytic stability, and selective solubilization, are proposed as key determinants for autocatalytic reproduction exploited in protometabolic evolution. This evolutionary process led from autocatalytic networks within preexisting compartments to discrete, reproducing, mobile vesicular protocells with the capacity to use soluble sugar phosphates and hence the opportunity to develop nucleic acids. Fidelity of information transfer in the reproduction of these increasingly complex autocatalytic networks is a key selection pressure in prebiological evolution that eventually leads to the selection of nucleic acids as a digital information subsystem and hence the emergence of fully functional chemotons capable of Darwinian evolution. 1 Introduction: Chemoton Subsystems and Evolutionary Pathways Living cells are autocatalytic entities that harness redox energy via the selective catalysis of biochemical transformations. -

Organic Chemistry Lesson 10 Reactions of Organic Molecules Combusition Reactions Substitution Reactions
Organic Chemistry Lesson 10 Reactions of Organic Molecules Combusition Reactions Substitution Reactions Physical Sciences Grade 12 1 Instructions Lesson 10 (16 April 2020) 1. Read ALL the slides in this document. 2. Homework: Just understand the theory for now. • If you have any questions, post them onto your WhatsApp group and your teacher will attend to them when (s)he has a chance. • Additional Resources: • Summary on pg.132 of your textbook. Physical Sciences Grade 12 2 Types of Reactions of Organic Compounds 1. Esterification: formation of an ester from an alcohol and a carboxylic acid. 2. Combustion Reactions (aka Oxidation): organic molecule + oxygen. 3. Substitution Reactions: one or more atoms are replaced by other atoms. (i) alkanes to alkyl halides; (ii) alcohols to alkyl halides; (iii) alkyl halides to alcohols 4. Addition Reactions: one or more atoms are added. (i) hydrogenation; (ii) halogenation; (iii) hydrohalogenation; (iv) hydration 5. Elimination Reactions: one or more atoms are removed. (i) dehydrohalogenation; (ii) dehydration; (iii) cracking of alkanes Physical Sciences Grade 12 3 1. Esterification • Already discussed (refer to earlier notes). Physical Sciences Grade 12 4 2. Combustion Reactions, i.e. Oxidation • A combustion reaction is the reaction of an organic molecule with oxygen. • Please note: “combustion” does NOT mean “explosion”. All combustion reactions release energy but most do so WITHOUT any explosion. • Alkanes – in the form of fossil fuels – are currently the main source of energy worldwide as their combustion is highly exothermic. • Examples: Burning wood for a braai. Propane combusting in a gas stove. Petrol combusting in a car engine. Physical Sciences Grade 12 5 2. -

Activity 33. Hydrohalogenation & Hydration of Alkynes
Chem 201, Fall 2010 Activity 33, M Nov 22 Activity 33. Hydrohalogenation & Hydration of Alkynes Alkynes undergo many of the same addition reactions that alkenes do, including additions that require carbocation intermediates. Different energy and geometry changes may be required for additions to an alkyne and an alkene so some surprising outcomes may occur with alkynes. Model 1. Addition of HX Hydrogen halides (HCl, HBr, HI) add to alkynes to make vinyl halides . A carbocation mechanism is followed when the reaction is performed in the dark in a peroxide-free solvent. This addition obeys Markovnikov’s rule. A free-radical-chain mechanism is followed when HBr addition is initiated by organic peroxides. This addition gives an anti-Markonikov product. Critical Thinking Questions 1. Draw the carbocations indicated by the two bouncy curved arrows (below). Based on the observation that the carbocation mechanism yields a Markovnikov product, circle the more stable carbocation. 2. (CI) Draw the free radicals indicated by the two competing pathways (below). Based on the observation that the free-radical mechanism yields an anti-Markovnikov product, circle the more stable free radical. 3. The cations and radicals in CTQ #1 and #2 are called vinyl cations and vinyl radicals , respectively. Based on the regioselectivities reported in Model 1, what effect do alkyl substituents at the charged/radical carbon have on the energies of these species? Is this consistent with, or opposed to, their effect on R 3C+ and R 3C• species? 4. The geometry of a vinyl cation can be anticipated using VSEPR. Count electron domains in the cation below and predict all of the HCC bond angles. -

Organic Chemistry PEIMS Code: N1120027 Abbreviation: ORGCHEM Number of Credits That May Be Earned: 1/2-1
Course: Organic Chemistry PEIMS Code: N1120027 Abbreviation: ORGCHEM Number of credits that may be earned: 1/2-1 Brief description of the course (150 words or less): Organic chemistry is an introductory course that is designed for the student who intends to continue future study in the sciences. The student will learn the concepts and applications of organic chemistry. Topics covered include aliphatic and aromatic compounds, alcohols, aldehydes, ketones, acids, ethers, amines, spectra, and stereochemistry. A brief introduction into biochemistry is also provided. The laboratory experiments will familiarize the student with the important laboratory techniques, specifically spectroscopy. Traditional high school chemistry courses focus on the inorganic aspects of chemistry whereas organic chemistry introduces the student to organic compounds and their properties, mechanisms of formations, and introduces the student to laboratory techniques beyond the traditional high school chemistry curriculum. Essential Knowledge and Skills of the course: (a) General requirements. This course is recommended for students in Grades 11-12. The recommended prerequisite for this course is AP Chemistry. (b) Introduction. Organic chemistry is designed to introduce students to the fundamental concepts of organic chemistry and key experimental evidence and data, which support these concepts. Students will learn to apply these data and concepts to chemical problem solving. Additionally, students will learn that organic chemistry is still evolving by reading about current breakthroughs in the field. Finally, students will gain appreciation for role that organic chemistry plays in modern technological developments in diverse fields, ranging from biology to materials science. (c) Knowledge and skills. (1) The student will be able to write both common an IUPAC names for the hydrocarbon. -

HISTORICAL ASPECTS of ORGANIC CHEMISTRY TEACHING (On the Example of Chemistry Direction of Higher Educational Institutions)
Journal of Critical Reviews ISSN- 2394-5125 Vol 7, Issue 13, 2020 CONCEPTUAL - HISTORICAL ASPECTS OF ORGANIC CHEMISTRY TEACHING (on the example of chemistry direction of Higher educational institutions) Rajabov Khudayor Madrimovich Uzbekistan, Khorasm region, Rajabov Khudayor Madrimovich, Doctor of philosophy (PhD) on pedagogical sciences, Urganch State University E-mail: [email protected] Received: 16.04.2020 Revised: 18.05.2020 Accepted: 13.06.2020 Abstract. The article reveals the historical approach on the teaching of organic chemistry in pedagogical universities. The development of modern trends and general competencies of students on the basis of historical approach, the creation of educational motivation, the focus on independent research and training of creative specialists are the most important directions. In this regard, it is important to improve the technology for organizing the educational process on the basis of historical principles in the system of training future chemistry teachers, to improve the mechanisms on the methodological support, to fulfill the system of competencies and cyclic diagnostics. Keywords: historical approach, organic chemistry, conceptual - historical aspects, professional competence, principle of historicism, methodology, self-improvement, innovation, non-historical, positive attitude, extracurricular activities. © 2020 by Advance Scientific Research. This is an open-access article under the CC BY license (http://creativecommons.org/licenses/by/4.0/) DOI: http://dx.doi.org/10.31838/jcr.07.13.129 -

Reactions of Alkenes and Alkynes
05 Reactions of Alkenes and Alkynes Polyethylene is the most widely used plastic, making up items such as packing foam, plastic bottles, and plastic utensils (top: © Jon Larson/iStockphoto; middle: GNL Media/Digital Vision/Getty Images, Inc.; bottom: © Lakhesis/iStockphoto). Inset: A model of ethylene. KEY QUESTIONS 5.1 What Are the Characteristic Reactions of Alkenes? 5.8 How Can Alkynes Be Reduced to Alkenes and 5.2 What Is a Reaction Mechanism? Alkanes? 5.3 What Are the Mechanisms of Electrophilic Additions HOW TO to Alkenes? 5.1 How to Draw Mechanisms 5.4 What Are Carbocation Rearrangements? 5.5 What Is Hydroboration–Oxidation of an Alkene? CHEMICAL CONNECTIONS 5.6 How Can an Alkene Be Reduced to an Alkane? 5A Catalytic Cracking and the Importance of Alkenes 5.7 How Can an Acetylide Anion Be Used to Create a New Carbon–Carbon Bond? IN THIS CHAPTER, we begin our systematic study of organic reactions and their mecha- nisms. Reaction mechanisms are step-by-step descriptions of how reactions proceed and are one of the most important unifying concepts in organic chemistry. We use the reactions of alkenes as the vehicle to introduce this concept. 129 130 CHAPTER 5 Reactions of Alkenes and Alkynes 5.1 What Are the Characteristic Reactions of Alkenes? The most characteristic reaction of alkenes is addition to the carbon–carbon double bond in such a way that the pi bond is broken and, in its place, sigma bonds are formed to two new atoms or groups of atoms. Several examples of reactions at the carbon–carbon double bond are shown in Table 5.1, along with the descriptive name(s) associated with each. -

UNIT-III: Mechanistic and Sterochemical Aspects of Addition Reaction Involving Electrophiles & Nucleophiles
MCH-204:ORGANIC CHEMISTRY UNIT-III: Mechanistic and sterochemical aspects of addition reaction Involving electrophiles & nucleophiles Prof.Anand Halve S.O.S in Chemistry Jiwaji University Gwalior ELECTROPHILIC ADDITION REACTIONS markovnikov’ additions markovnikov’s rule Addition of hydrogen to an unsymmetrical olefin occurs at those carbon atoms with maximum number of hydrogen atoms. (i.e., the carbon with least substitution). Electronegative group goes to more substituted carbon atom. Such an addition leads to a stabler carbocation. Such a reaction may lead to constitutional isomers but actually one of the products is formed as major product. X X Formed H HX X=F, Cl. X Olefin Not formed origin … X HX X X=F, Cl . carbocation is more stablised in T.S. Stereo specific product H X X carbocation is not enough stablilised in transition state Olefin Consider two possible sites for hydrogen addition (i) terminal or (ii) internal (substituted carbon). The addition of hydrogen at the terminal carbon leads to better stabilization of carbocation, the chances of stabilization increases with increase in conjugation with olefin. The terminal carbocation require higher activation energy which is not a favorable condition, leading to slower reaction rate. However, the generation of non terminal carbocation is assisted by hyperconjugative stabilization leading to a lower activation energy. Alkenes-some facts Due to trigonal planar geometry of olefin carbon atoms the addition can occur on the same side (syn periplanar) or on opposite sides (anti periplanar). Alkenes are generally nucleophilic. The C=C double bond provides a higher energy HOMO (highest occupied molecular orbitals). Electron donating groups increase the rate for electrophilic attack as they assist in carbocation and positive charge stabilization in the TS. -

Che 360: Polymer Chemistry Syllabus
CHE 360: POLYMER CHEMISTRY SYLLABUS Instructor: Dr. Zachary Rodgers Text: Introduction to Polymer Chemistry (Carraher) Course webpage: Desire2Learn Prerequisites: CHE 262: Organic Chemistry Contact Info: (724)-946-6289 [email protected] Class Time: TR 9:10 – 10:50 am Lab Time: R 2:00 – 5:00 pm Course Overview The word polymer comes from the Greek words “poly”, meaning many, and “mer”, meaning parts. Like the macrostructures we see every day, such as a house built of brick, the larger structures of polymers have very different properties from their smaller chemical building blocks. The uniqueness of polymer properties provide most of the materials we interact with on a regular basis, including our clothes, plastics, paper, adhesives, and even our bodies themselves. Therefore, no education in chemistry would be complete without describing the general properties, synthesis, processing, and function of polymeric materials. This semester long course aims to: Describe how polymer morphology affects a polymer’s overall properties and behavior Teach polymer characterization techniques to determine their structure and size Detail a variety of polymerization mechanisms and their underlying kinetic/thermodynamic characteristics Introduce the practical techniques used to synthesize a wide range of polymeric structures Provide several examples of the industrial processing techniques used to generate consumable polymer products Make connections between material to answer complex and multi-step problems Expose students to literature research in materials and polymer science Grading Grading Weights 10% Homework Assignments (~6 – 8 total) 20% Laboratory Grade 37.5% Exams (3 Tests) 12.5% Student Presentation 20% Final Grading Scale 90 - 100 A 72 – 77 C 88 - 89 B+ 70 – 71 C- 82 – 87 B 68 – 69 D+ 80 – 81 B- 60 – 67 D 78 – 79 C+ < 60 F The scale set in this syllabus is non-negotiable. -
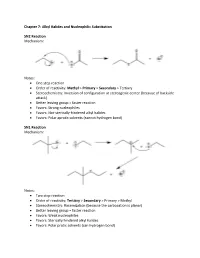
Alkyl Halides and Nucleophilic Substitution SN2 Reaction
Chapter 7: Alkyl Halides and Nucleophilic Substitution SN2 Reaction Mechanism: Notes: • One step reaction • Order of reactivity: Methyl > Primary > Secondary > Tertiary • Stereochemistry: Inversion of configuration at stereogenic center (because of backside attack) • Better leaving group = faster reaction • Favors: Strong nucleophiles • Favors: Not-sterically-hindered alkyl halides • Favors: Polar aprotic solvents (cannot hydrogen bond) SN1 Reaction Mechanism: Notes: • Two step reaction • Order of reactivity: Tertiary > Secondary > Primary > Methyl • Stereochemistry: Racemization (because the carbocation is planar) • Better leaving group = faster reaction • Favors: Weak nucleophiles • Favors: Sterically hindered alkyl halides • Favors: Polar protic solvents (can hydrogen bond) Important Trends Chapter 8: Alkyl Halides and Elimination Reactions E2 Reaction Mechanism: Notes: • One step reaction • Order of reactivity: Tertiary > Secondary > Primary • Stereochemistry: antiperiplanar arrangement of H and X • Better leaving group = faster reaction • Favors: Polar aprotic solvents, strong bases • Products follow Zaitsev rule (more substituted alkene is the major product) E1 Reaction Mechanism: Notes: • Two step reaction • Order of reactivity: Tertiary > Secondary > Primary • Stereochemistry: Trigonal planar carbocation intermediate • Better leaving group = faster reaction • Favors: Polar protic solvents, weak bases • Products follow Zaitsev rule Chapter 9: Alcohols, Ethers, and Epoxides Preparation of Alcohols Mechanism: Notes: • SN2 mechanism -

Introduction to Theory of Chemical Reactions
INTRODUCTION TO THEORY OF CHEMICAL REACTIONS BACKGROUND The introductory part of the organic chemistry course has three major modules: Molecular architecture (structure), molecular dynamics (conformational analysis), and molecular transformations (chemical reactions). An understanding of the first two is crucial to an understanding of the third one. The rest of the organic chemistry course will be largely spent on studying different kinds of reactions and mechanisms. A summary of key concepts follows. 1. MOLECULAR ARCHITECTURE - Basic principles of molecular structure. a) Atomic structure b) Orbitals and hybridization c) Covalent bonding d) Lewis structures and resonance forms e) Isomerism, structural and geometric isomers f) Polarity, functional groups, nomenclature systems g) Three-dimensional structures, stereochemistry, stereoisomers. 2. MOLECULAR DYNAMICS - Basic principles of molecular motion involving rotation around single bonds and no bond breakage. The focus is on conformations and their energy relationships, especially in reference to alkanes and cycloalkanes (conformational analysis). a) Steric interactions b) Torsional strain and Newman projections c) Angle strain in cycloalkanes d) Conformations of cyclohexane and terminology associated with it. 3. MOLECULAR TRANSFORMATIONS - This is the part that comprises the bulk of organic chemistry courses. It is the study of chemical reactions and the principles that rule transformations. There are three major aspects of this module. In organic chemistry I we will focus largely on the first two, and leave the study of synthetic strategy for later. a) Reaction mechanisms - Step by step accounts of how electron movement takes place when bonds are broken and formed, and the conditions that favor these processes (driving forces). An understanding of the basic concepts of thermodynamics and kinetics is important here. -

Organic Chemistry I: Reactions and Overview
Organic Chemistry I: Reactions and Overview Andrew Rosen Editor: Raghav Malik January 13, 2013 Contents I Library of Synthetic Reactions 3 II Organic Trends and Essentials 4 1 The Basics: Bonding and Molecular Structure 4 1.1 Resonance Stability . 4 2 Families of Carbon Compounds 4 2.1 Strength of London Dispersion Forces (Polarizability) . 4 2.2 Degree of Unsaturation . 4 3 An Introduction to Organic Reactions and Their Mechanisms 4 3.1 Comparing Acid Strengths . 4 4 Nomenclature and Conformations of Alkanes and Cycloalkanes 5 4.1 Ring Flipping . 5 5 Stereochemistry 5 5.1 Naming Enantiomers via the -R and -S System . 5 5.2 Stereochemistry Examples . 6 6 Ionic Reactions - Overview 6 6.1 General Nucleophilic Substitution Reactions . 6 6.2 Carbocation Stability . 6 6.3 Factors Aecting the Rates of SN 1 and SN 2 Reactions . 6 6.4 Elimination Reactions . 7 6.5 Summary . 7 7 Alkenes and Alkynes I - Overview 8 7.1 The E-Z System . 8 7.2 Relative Stabilities of Alkenes . 8 7.3 Factors Aecting Elimination Reactions . 8 7.4 Acid-Catalyzed Dehydration of Alcohols . 8 1 III Reaction Mechanisms 9 8 Ionic Reactions - Mechanisms 9 8.1 The SN 2 Reaction . 9 8.2 The SN 1 Reaction . 10 8.3 The E2 Reaction . 10 8.4 The E1 Reaction . 11 9 Alkenes and Alkynes I - Mechanisms 11 9.1 Acid-Catalyzed Dehydration of Secondary or Tertiary Alcohols: An E1 Reaction . 11 9.2 Acid-Catalyzed Dehydration of Primary Alcohols: An E2 Reaction . 12 9.3 Synthesis of Alkynes from Vic-Dihalides . -

Introduction to Biology. Lecture 6
Where we are? Origin of life Introduction to Biology. Lecture 6 Alexey Shipunov Minot State University September 5, 2012 Shipunov Introduction to Biology. Lecture 6 Where we are? Origin of life Outline 1 Where we are? 2 Origin of life Molecules of life Primordial living structures Alternatives and amendments to abiogenesis Shipunov Introduction to Biology. Lecture 6 Where we are? Origin of life Outline 1 Where we are? 2 Origin of life Molecules of life Primordial living structures Alternatives and amendments to abiogenesis Shipunov Introduction to Biology. Lecture 6 Where we are? Origin of life Evolution is the fact and research program Given the amount of evidence presented, evolution is a fact Evolution is also an extremely useful, working research program, both in biology and medicine Shipunov Introduction to Biology. Lecture 6 Molecules of life Where we are? Primordial living structures Origin of life Alternatives and amendments to abiogenesis Origin of life Molecules of life Shipunov Introduction to Biology. Lecture 6 Molecules of life Where we are? Primordial living structures Origin of life Alternatives and amendments to abiogenesis Organic chemistry: chemistry of carbon Carbon skeleton And H, O, N, P, S Shipunov Introduction to Biology. Lecture 6 Molecules of life Where we are? Primordial living structures Origin of life Alternatives and amendments to abiogenesis Four types of biomolecules Lipids: hydrophobic Carbohydrates (sugars): multiple −OH groups Amino acids: N + C + O and hydrogen Nucleotides: cycle with nitrogen (heterocycle), sugar and phosphoric acid Shipunov Introduction to Biology. Lecture 6 Molecules of life Where we are? Primordial living structures Origin of life Alternatives and amendments to abiogenesis Organic polymers Polymeric carbohydrates: polysaccharides (like cellulose and starch) Polymeric amino acids: proteins Polymeric nucleotides: nucleic acids (DNA and RNA) Shipunov Introduction to Biology.