TPN Electrolytes (Multiple Electrolyte Additive)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chemistry Lab Hygiene Plan
UNIVERSITY OF MAINE Department of Chemistry CHEMICAL HYGIENE PLAN October, 2001 Contents I. Personal Protective Equipment .. 3 II. Storage of Chemicals .. 6 III. Disposal of Chemicals .. 16 IV. Reading MSDS .. 19 V. Emergencies and the Emergency Action Plan .. 26 VI. Standard Operating Guidelines .. 33 VII. General Housekeeping and Prudent Practices .. 40 2 I. Laboratory Clothing and Personal Protective Equipment A. Dressing for safety in the laboratory Individuals should prepare for a safe laboratory experience by dressing appropriately for laboratory work. Appropriate clothing includes the following: • Shoes should fully cover the feet to protect against spills; no open-toed shoes or sandals are permitted, and shoes with mesh inserts (such as athletic shoes) are not recommended. One may choose to keep a pair of sturdy leather shoes in the laboratory to change into upon arrival. • Trousers or skirts falling below the knee are preferred; if shorter garments are worn, a lab coat or apron of below knee length is required. Preferred materials are resistant polyester, cotton or wool, since ordinary polyester and acrylics may be dissolved by common laboratory solvents. • Neckties, if worn, should be firmly clipped to the shirt or confined inside a lab coat or apron. • Loose, flowing garments and scarves should be avoided; they may easily pick up spills or trail through a burner flame. • In a laboratory where open flames may be used, long hair should be confined. • Loose jewelry should be avoided, since it may catch on equipment. Also avoid ornate rings that can damage protective gloves or make wearing or removing gloves difficult B. Protective Equipment Every laboratory must have available, and workers must be trained in the use of, safety goggles, face masks, lab coats or aprons, gloves, and reaction shields. -

Transport of Dangerous Goods
ST/SG/AC.10/1/Rev.16 (Vol.I) Recommendations on the TRANSPORT OF DANGEROUS GOODS Model Regulations Volume I Sixteenth revised edition UNITED NATIONS New York and Geneva, 2009 NOTE The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. ST/SG/AC.10/1/Rev.16 (Vol.I) Copyright © United Nations, 2009 All rights reserved. No part of this publication may, for sales purposes, be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, electrostatic, magnetic tape, mechanical, photocopying or otherwise, without prior permission in writing from the United Nations. UNITED NATIONS Sales No. E.09.VIII.2 ISBN 978-92-1-139136-7 (complete set of two volumes) ISSN 1014-5753 Volumes I and II not to be sold separately FOREWORD The Recommendations on the Transport of Dangerous Goods are addressed to governments and to the international organizations concerned with safety in the transport of dangerous goods. The first version, prepared by the United Nations Economic and Social Council's Committee of Experts on the Transport of Dangerous Goods, was published in 1956 (ST/ECA/43-E/CN.2/170). In response to developments in technology and the changing needs of users, they have been regularly amended and updated at succeeding sessions of the Committee of Experts pursuant to Resolution 645 G (XXIII) of 26 April 1957 of the Economic and Social Council and subsequent resolutions. -

Notable Reactivity of Acetonitrile Towards Li2o2/Lio2 Probed By
Topics in Catalysis https://doi.org/10.1007/s11244-018-1072-5 ORIGINAL ARTICLE Notable Reactivity of Acetonitrile Towards Li2O2/LiO2 Probed by NAP XPS During Li–O2 Battery Discharge Tatiana K. Zakharchenko1 · Alina I. Belova1 · Alexander S. Frolov1 · Olesya O. Kapitanova1 · Juan‑Jesus Velasco‑Velez2 · Axel Knop‑Gericke2,5 · Denis Vyalikh3,4 · Daniil M. Itkis1 · Lada V. Yashina1 © Springer Science+Business Media, LLC, part of Springer Nature 2018 Abstract One of the key factors responsible for the poor cycleability of Li–O2 batteries is a formation of byproducts from irreversible reactions between electrolyte and discharge product Li 2O2 and/or intermediate LiO2. Among many solvents that are used as electrolyte component for Li–O2 batteries, acetonitrile (MeCN) is believed to be relatively stable towards oxidation. Using near ambient pressure X-ray photoemission spectroscopy (NAP XPS), we characterized the reactivity of MeCN in the Li–O2 battery. For this purpose, we designed the model electrochemical cell assembled with solid electrolyte. We discharged it first in O2 and then exposed to MeCN vapor. Further, the discharge was carried out in O2 + MeCN mixture. We have dem- onstrated that being in contact with Li–O2 discharge products, MeCN oxidizes. This yields species that are weakly bonded to the surface and can be easily desorbed. That’s why they cannot be detected by the conventional XPS. Our results suggest that the respective chemical process most probably does not give rise to electrode passivation but can decrease considerably the Coulombic efficiency of the battery. Keywords Li–O2 battery · In situ NAP XPS · Acetonitrile · Side reactions 1 Introduction Li–O2 batteries promise extraordinary high specific energy that makes them interesting for the next generation power technologies [1, 2]. -

Chemical Chemical Hazard and Compatibility Information
Chemical Chemical Hazard and Compatibility Information Acetic Acid HAZARDS & STORAGE: Corrosive and combustible liquid. Serious health hazard. Reacts with oxidizing and alkali materials. Keep above freezing point (62 degrees F) to avoid rupture of carboys and glass containers.. INCOMPATIBILITIES: 2-amino-ethanol, Acetaldehyde, Acetic anhydride, Acids, Alcohol, Amines, 2-Amino-ethanol, Ammonia, Ammonium nitrate, 5-Azidotetrazole, Bases, Bromine pentafluoride, Caustics (strong), Chlorosulfonic acid, Chromic Acid, Chromium trioxide, Chlorine trifluoride, Ethylene imine, Ethylene glycol, Ethylene diamine, Hydrogen cyanide, Hydrogen peroxide, Hydrogen sulfide, Hydroxyl compounds, Ketones, Nitric Acid, Oleum, Oxidizers (strong), P(OCN)3, Perchloric acid, Permanganates, Peroxides, Phenols, Phosphorus isocyanate, Phosphorus trichloride, Potassium hydroxide, Potassium permanganate, Potassium-tert-butoxide, Sodium hydroxide, Sodium peroxide, Sulfuric acid, n-Xylene. Acetone HAZARDS & STORAGE: Store in a cool, dry, well ventilated place. INCOMPATIBILITIES: Acids, Bromine trifluoride, Bromine, Bromoform, Carbon, Chloroform, Chromium oxide, Chromium trioxide, Chromyl chloride, Dioxygen difluoride, Fluorine oxide, Hydrogen peroxide, 2-Methyl-1,2-butadiene, NaOBr, Nitric acid, Nitrosyl chloride, Nitrosyl perchlorate, Nitryl perchlorate, NOCl, Oxidizing materials, Permonosulfuric acid, Peroxomonosulfuric acid, Potassium-tert-butoxide, Sulfur dichloride, Sulfuric acid, thio-Diglycol, Thiotrithiazyl perchlorate, Trichloromelamine, 2,4,6-Trichloro-1,3,5-triazine -

Hazardous Materials Safety Administration 49 CFR Parts 171, 172, 173, Et Al
Vol. 80 Thursday, No. 5 January 8, 2015 Part II Department of Transportation Pipeline and Hazardous Materials Safety Administration 49 CFR Parts 171, 172, 173, et al. Hazardous Materials: Harmonization With International Standards (RRR); Final Rule VerDate Sep<11>2014 19:02 Jan 07, 2015 Jkt 235001 PO 00000 Frm 00001 Fmt 4717 Sfmt 4717 E:\FR\FM\08JAR2.SGM 08JAR2 mstockstill on DSK4VPTVN1PROD with RULES2 1076 Federal Register / Vol. 80, No. 5 / Thursday, January 8, 2015 / Rules and Regulations DEPARTMENT OF TRANSPORTATION III. Incorporation by Reference Discussion reduces regulatory compliance costs and Under 1 CFR Part 51 helps to avoid costly frustrations of Pipeline and Hazardous Materials IV. Comment Discussion international shipments. PHMSA’s Safety Administration V. Section-by-Section Review continued leadership in maintaining VI. Regulatory Analyses and Notices A. Statutory/Legal Authority for the consistency with international 49 CFR Parts 171, 172, 173, 175, 176, Rulemaking regulations enhances the hazardous 178 and 180 B. Executive Orders 12866 and 13563 and materials safety program and assists in DOT Regulatory Policies and Procedures maintaining a favorable trade balance. [Docket Nos. PHMSA–2013–0260 (HM– C. Executive Order 13132 215M)] D. Executive Order 13175 II. Background E. Regulatory Flexibility Act, Executive RIN 2137–AF05 PHMSA published a notice of Order 13272, and DOT Policies and proposed rulemaking (NPRM) under Procedures Docket HM–215M (79 FR 50741, August Hazardous Materials: Harmonization F. Paperwork Reduction Act With International Standards (RRR) G. Regulatory Identifier Number (RIN) 25, 2014) to incorporate various H. Unfunded Mandates Reform Act amendments to harmonize the HMR AGENCY: Pipeline and Hazardous I. -

Chemical Names and CAS Numbers Final
Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number C3H8O 1‐propanol C4H7BrO2 2‐bromobutyric acid 80‐58‐0 GeH3COOH 2‐germaacetic acid C4H10 2‐methylpropane 75‐28‐5 C3H8O 2‐propanol 67‐63‐0 C6H10O3 4‐acetylbutyric acid 448671 C4H7BrO2 4‐bromobutyric acid 2623‐87‐2 CH3CHO acetaldehyde CH3CONH2 acetamide C8H9NO2 acetaminophen 103‐90‐2 − C2H3O2 acetate ion − CH3COO acetate ion C2H4O2 acetic acid 64‐19‐7 CH3COOH acetic acid (CH3)2CO acetone CH3COCl acetyl chloride C2H2 acetylene 74‐86‐2 HCCH acetylene C9H8O4 acetylsalicylic acid 50‐78‐2 H2C(CH)CN acrylonitrile C3H7NO2 Ala C3H7NO2 alanine 56‐41‐7 NaAlSi3O3 albite AlSb aluminium antimonide 25152‐52‐7 AlAs aluminium arsenide 22831‐42‐1 AlBO2 aluminium borate 61279‐70‐7 AlBO aluminium boron oxide 12041‐48‐4 AlBr3 aluminium bromide 7727‐15‐3 AlBr3•6H2O aluminium bromide hexahydrate 2149397 AlCl4Cs aluminium caesium tetrachloride 17992‐03‐9 AlCl3 aluminium chloride (anhydrous) 7446‐70‐0 AlCl3•6H2O aluminium chloride hexahydrate 7784‐13‐6 AlClO aluminium chloride oxide 13596‐11‐7 AlB2 aluminium diboride 12041‐50‐8 AlF2 aluminium difluoride 13569‐23‐8 AlF2O aluminium difluoride oxide 38344‐66‐0 AlB12 aluminium dodecaboride 12041‐54‐2 Al2F6 aluminium fluoride 17949‐86‐9 AlF3 aluminium fluoride 7784‐18‐1 Al(CHO2)3 aluminium formate 7360‐53‐4 1 of 75 Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number Al(OH)3 aluminium hydroxide 21645‐51‐2 Al2I6 aluminium iodide 18898‐35‐6 AlI3 aluminium iodide 7784‐23‐8 AlBr aluminium monobromide 22359‐97‐3 AlCl aluminium monochloride -

Material Safety Data Sheets and Their Relevance to Customs Work
Material Safety Data Sheets and their relevance to Customs work Updated as on 18.09.2015 WCO Programme Global Shield (PGS) – E-book No.05 [Training Material for Departmental Use] E-BOOK On Material Safety Data Sheets Material Safety Data Sheets and their relevance to Customs work Note: 1. In this E-book, attempts have been made to make the officers aware about Material Safety Data Sheets and their utility at the time of handling, examination and storage of various chemicals. This will also help in proper classification of chemicals under Customs Tariff. 2. Though all efforts have been made to make this document error free, it is possible that some errors might have crept into the document. If you notice any errors, the same may be brought to the notice of the NACEN, RTI, Kanpur on the Email address: [email protected]. This may not be a perfect E-book. If you have any suggestion to improve this book, you are requested to forward the same to us. 3. This e-book is one of the several e-books dealing with different aspects of WCO Programme Global Shield (PGS). The Programme Global Shield (PGS) is a long term law enforcement initiative of WCO alongwith its partner organizations, namely, United Nations Office on Drug and Crime (UNODC), International Police Organization (INTERPOL) and member countries. This Programme is aimed at combating the illicit diversion and trafficking of high risk precursor chemicals, which are commonly used by criminal elements/terrorist organizations to make Improvised Explosive Devices (IEDs). 4. It is acknowledged here that in preparing this e-book, the WCO training material as well as material from other sources including that available freely on internet have been used. -
![United States Patent '[19]](https://docslib.b-cdn.net/cover/0035/united-states-patent-19-3350035.webp)
United States Patent '[19]
United States Patent ‘[19] [11] 4,441,923 Swanson [45] Apr. 10, 1984 {54] INTEGRATED PROCESS USING 3,938,988 2/1976 Othmer ............................. ..75/10R NON-STOICHIOMETRIC SULFIDES OR OXIDES OF POTASSIUM FOR MAKING FOREIGN PATENT DOCUMENTS LESS ACTIVE METALS AND 590274 7/ 1947 United Kingdom .................. .. 75/66 HYDROCARBONS OTHER PUBLICATIONS [76] Inventor: Rollan Swanson, 220 California St., Mellor, “A Comprehensive Treatise on Inorganic and Santa Monica, Calif. 90403 Theoretical Chemistry”, 1922, pp. 445-451. [21] Appl. No.: 343,977 “Comprehensive Inorganic Chemistry”, Pergamon Press, 1973, pp. 371-373. [22] Filed: Jan. 29, 1982 Primary Examiner—Edward J. Meros Related US. Application Data Attorney, Agent, or Firm—-Albert F. Kronman [63] Continuation of Ser. No. 169,281, Jul. 16, 1980, aban [57] ABSTRACT doned, which is a continuation-in-part of Ser. No. Disclosed is a combinative integrated chemical process 706,795, Jul. 19, 1976, abandoned, and Ser. No. 3,590, Jan. 15, 1979, abandoned. using inorganic reactants and yielding, if desired, or ganic products. The process involves ?rst the produc [51] Int. Cl.3 ...................... .. C21B 15/00; C22B 5/02; tion of elemental potassium by the thermal or thermal C22B 26/10 reduced pressure decomposition of potassium oxide or [52] US. Cl. ..................................... .. 75/28; 75/20 R; potassium sul?de and distillation of the potassium. This 75/66; 75/67 R; 75/69; 75/71; 75/72; 75/77; elemental potassium is then used to reduce ores or ore 75/86; 423/414; 423/560; 423/561 A; 423/567 concentrates of copper, zinc, lead, magnesium, cad A; 423/641; 423/657; 585/500; 585/534; mium, iron, arsenic, antimony or silver to yield one or 585/638; 585/700; 585/733 more of these less active metals in elemental form. -

1 Draft Chemicals (Management and Safety)
Draft Chemicals (Management and Safety) Rules, 20xx In exercise of the powers conferred by Sections 3, 6 and 25 of the Environment (Protection) Act, 1986 (29 of 1986), and in supersession of the Manufacture, Storage and Import of Hazardous Chemical Rules, 1989 and the Chemical Accidents (Emergency Planning. Preparedness and Response) Rules, 1996, except things done or omitted to be done before such supersession, the Central Government hereby makes the following Rules relating to the management and safety of chemicals, namely: 1. Short Title and Commencement (1) These Rules may be called the Chemicals (Management and Safety) Rules, 20xx. (2) These Rules shall come into force on the date of their publication in the Official Gazette. Chapter I Definitions, Objectives and Scope 2. Definitions (1) In these Rules, unless the context otherwise requires (a) “Act” means the Environment (Protection) Act, 1986 (29 of 1986) as amended from time to time; (b) “Article” means any object whose function is determined by its shape, surface or design to a greater degree than its chemical composition; (c) “Authorised Representative” means a natural or juristic person in India who is authorised by a foreign Manufacturer under Rule 6(2); (d) “Chemical Accident” means an accident involving a sudden or unintended occurrence while handling any Hazardous Chemical, resulting in exposure (continuous, intermittent or repeated) to the Hazardous Chemical causing death or injury to any person or damage to any property, but does not include an accident by reason only -

Defect Chemistry in Alkali Peroxides and Superoxides
Defect Chemistry in Alkali Peroxides and Superoxides Von der Fakultät Chemie der Universität Stuttgart zur Erlangung der Würde eines Doktors der Naturwissenschaften (Dr. rer. nat.) genehmigte Abhandlung Vorgelegt von Oliver Gerbig aus Darmstadt Hauptberichter: Prof. Dr. J. Maier Mitberichter: Prof. Dr. J. Bill Prüfungsvorsitzender: Prof. Dr. J. van Slageren Tag der Einreichung: 28. Februar 2014 Tag der mündlichen Prüfung: 7. Mai 2014 Max-Planck-Institut für Festkörperforschung Stuttgart 2014 Erklärung Die vorliegende Doktorarbeit wurde vom Autor selbst in der Abteilung von Prof. J. Maier am Max- Planck-Institut für Festkörperforschung im Zeitraum von Januar 2010 bis März 2014 angefertigt. Der Inhalt ist die eigene Arbeit des Autors, Ausnahmen sind gekennzeichnet und wurden noch nicht zur Erlangung einer Qualifizierung oder eines Titels an der einer akademischen Institution eingereicht. Stuttgart, den 28. Februar 2014 Oliver Gerbig Declaration The work described in this thesis was carried out by the author in the Department of Prof. J. Maier at the Max Planck Institute for Solid State Research from January 2010 to March 2014. The content is the original work of the author except where indicated otherwise and has not been previously submitted to obtain any other degree or qualification at any academic institution. Stuttgart, 28th February 2014 Oliver Gerbig Table of Contents 1 Introduction and Motivation ........................................................................................................... 1 1.1 Inorganic Peroxides and -

Potassium Peroxide Pop
POTASSIUM PEROXIDE POP CAUTIONARY RESPONSE INFORMATION 4. FIRE HAZARDS 7. SHIPPING INFORMATION 4.1 Flash Point: 7.1 Grades of Purity: Commercial; Pure Common Synonyms Solid (powder) Yellow Odorless Not pertinent 7.2 Storage Temperature: Ambient Potassium superoxide 4.2 Flammable Limits in Air: Not pertinent 7.3 Inert Atmosphere: No requirement 4.3 Fire Extinguishing Agents: Flood with Sinks and mixes violently with water. 7.4 Venting: Pressure-vacuum water from a protected area. 7.5 IMO Pollution Category: Currently not available Evacuate. 4.4 Fire Extinguishing Agents Not to Be Keep people away. Avoid contact with solid and dust. Used: A small amount of water may 7.6 Ship Type: Currently not available Shut off ignition sources and call fire department. cause explosions. 7.7 Barge Hull Type: Currently not available Notify local health and pollution control agencies. 4.5 Special Hazards of Combustion Protect water intakes. Products: Currently not available 8. HAZARD CLASSIFICATIONS 4.6 Behavior in Fire: Increases intensity of Not flammable. fire and can start fires when in contact 8.1 49 CFR Category: Oxidizer Fire Will increase the intensity of a fire. with organic combustibles 8.2 49 CFR Class: 5.1 May cause fire on contact with combustibles. 4.7 Auto Ignition Temperature: Not pertinent Combat fires from safe distance or protected location. 8.3 49 CFR Package Group: I Flood discharge area with water. 4.8 Electrical Hazards: Not pertinent 8.4 Marine Pollutant: No 4.9 Burning Rate: Not pertinent 8.5 NFPA Hazard Classification: Exposure CALL FOR MEDICAL AID. 4.10 Adiabatic Flame Temperature: Currently DUST not available Category Classification Irritating to eyes, nose and throat. -
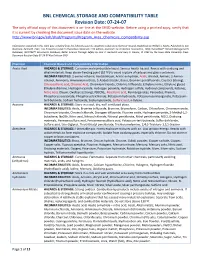
BNL CHEMICAL STORAGE and COMPATIBILITY TABLE Revision Date: 07-24-07 the Only Official Copy of This Document Is On-Line at the SHSD Website
BNL CHEMICAL STORAGE AND COMPATIBILITY TABLE Revision Date: 07-24-07 The only official copy of this document is on-line at the SHSD website. Before using a printed copy, verify that it is current by checking the document issue date on the website. http://www.bnl.gov/esh/shsd/Programs/Program_Area_Chemicals_Compatibility.asp Information contained in this table was compiled from the following sources: Academic Laboratory Chemical Hazards Guidebook by William J. Mahn, Published by Van Nostrand, Reinhold, 1991; Fire Protection Guide to Hazardous Materials 11th edition, National Fire Protection Association, 1994; Hazardtext® Hazard Managements Database; INFOTEXT® Documents Database; Better Science Through Safety by Jack A. Gerlovich and Gary E. Downs, © 1981 by the Iowa State University Press. Document Revision Date 07-24-07 Ken Erickson CHO Chemical Chemical Hazard and Compatibility Information Acetic Acid HAZARDS & STORAGE: Corrosive and combustible liquid. Serious health hazard. Reacts with oxidizing and alkali materials. Keep above freezing point (62 ºF) to avoid rupture of carboys and glass containers. INCOMPATIBILITIES: 2-amino-ethanol, Acetaldehyde, Acetic anhydride, Acids, Alcohol, Amines, 2-Amino- ethanol, Ammonia, Ammonium nitrate, 5-Azidotetrazole, Bases, Bromine pentafluoride, Caustics (strong), Chlorosulfonic acid, Chromic Acid, Chromium trioxide, Chlorine trifluoride, Ethylene imine, Ethylene glycol, Ethylene diamine, Hydrogen cyanide, Hydrogen peroxide, Hydrogen sulfide, Hydroxyl compounds, Ketones, Nitric Acid, Oleum, Oxidizers