Department Botany, Abasaheb Garware College, Pune 411 004
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
SUNITI UDAYAN PATHAK UGC-NET (JRF), Ph
SUNITI UDAYAN PATHAK UGC-NET (JRF), Ph. D. Assistant Professor, Department of Biotechnology, MES Abasaheb Garware College, Pune [email protected] Teaching Experience: Undergraduate Nature of Institution Period Position held Appointment Subject M I T Lecturer Visiting Biochemistry 1 Academic College of B.Pharm Theory & Year Pharmacy, Practical (2000-01) Paud Road, Pune Sinhgad Lecturer Visiting Biochemistry 2 Academic College of B.Sc. Nursing Theory Years Nursing, (2005-06& Pune 2006-2007) Maharshi Lecturer Visiting Biochemistry 1 Academic Karve Stree B.Sc. Nursing Theory year (2006- Shikshan 2007; 2007- Sanstha 2008) College of Nursing Abasaheb Lecturer B.Sc Leave Molecular 2nd Semester Garware (Zoology) Vacancy Biology (2007) College, Karve Rd, Pune Vasantdada Lecturer Visiting Biochemistry 1st Semester Sugar PG Diploma Theory (2007) Institute, (sugar Tech. & Pune Alcohol Tech.) MES Lecturer Permanent Cell Biology. 2008- till date Abasaheb Dept. of Zoology, Garware Biotechnology Molecular College Biology, Metabolic Pathways Teaching Experience: Post-Graduate Nature of Institution Position held Appointment Subject Period Modern Post Biochemistry, Cell & 2 yrs College, Graduate Temporary Molecular Biology (1994 –96) Shivaji Lecturer in Nagar, Pune Zoology Cell Biology, 10 yrs. Metabolic pathways, (2008 till date) MES, Assistant Permanent Proteomics, Garware Professor Molecular Biology College Research Experience ➢ Project Associate on UGC Major Research Project - “Studies on in vivo glycation of Alkaline phosphatase in normal and diabetic rats: beneficial effects of amino functional groups and herbal extracts on glycation”. Project period August 2001 to July 2004 at Division of Biochemistry, Department of Chemistry, University of Pune, Pune. ➢ BCUD Project (2016-18): Investigations on the effect of high dietary fructose on protein glycation in Drosophila and evaluation of herbal antiglycating agents for prevention of sugar-induced damage. -

AQAR 2012-13 Page 1 1.6 Date of Establishment of 01 / 06 / 2002 IQAC
Maharashtra Education Society’s ABASAHEB GARWARE COLLEGE Karve Road, Pune 411004, Maharashtra State. ANNUAL QUALITY ASSURANCE REPORT (AQAR) FOR THE ACADEMIC YEAR 2012-13 Submitted to: National Accreditation and Assessment Council (NAAC) P.O. Box No. 1075, Nagarbhavi, Bangaluru, 560072 India The Annual Quality Assurance Report (AQAR) of the IQAC 2012-13 Part – A 1. Details of the Institution 1.1 Name of the MES Abasaheb Garware College Institution: 1.2 Address Karve Road. City / Town Pune State Maharashtra Pin Code 411004 Institutional email [email protected] address Contact Nos. 020-41038201; 020-41038200 Name of the Head Dr. Shrikant G. Gupta of the Institution Te. No. With STD 020-41038201 Code Mobile 91-9881300984 Name of the IQAC Dr. B. D. Bhole. Coordinator Mobile 9970302467 IQAC email address [email protected] 1.3 NAAC Track ID (For ex. MHCOGN 18879) 1.4 Website address: www.mesgarwarecollege.org Web-link of the http://www.mesgarwarecollege.org/IQAC/AQAR201213.pdf AQAR: 1.5 Accreditation Details: Year of Validity Sr. No. Cycle Grade CGPA Accreditation Period 1 1st Cycle B+ - 2004 2004-10 2 2nd Cycle A 3.15 2010 2010-15 MES Abasaheb Garware College AQAR 2012-13 Page 1 1.6 Date of Establishment of 01 / 06 / 2002 IQAC: 1.7 AQAR for the year: 2012-13 1.8 Details of the previous year’s AQAR submitted to NAAC after the latest Assessment and Accreditation by NAAC: i) AQAR 2010-11 18.11.2011 ii) AQAR 2011-12 28.09.2012 1.9 Institutional Status University State Central Deemed Private Affiliated College Yes No Constituent College Yes No Autonomous College Yes No of UGC Regulatory Agency Yes No approved Institution Type of Institution Co- Men Women education Urban Rural Tribal Financial Status Grant-in-aid UGC 2(f) UGC 12B Grant-in-aid + Self-financing Totally self-financing 1.10 Type of Faculty / Program Arts Science Commerce Law PEI (Phy.Edu.) TEI Engineering Health Management (Edu) Science Others MES Abasaheb Garware College AQAR 2012-13 Page 2 1.11 Name of the Affiliating University University of Pune, Pune. -

Biotechnology – Admission
ry ,VIAHARASHTRA EDUCATION SOCIEff S (stN€E 1850) ABASAHEB GARWARE COLTEGE ffiB Karve Road, Deccan Gymkhana, Pune - 411004, Maharashtra, lndia I ph.: 020-4.1039200 PLATINUM JUBILEE E-mail: [email protected] I rvww.garwarecollege.mespune.in Prof. (Dr.) P. B. Buchade . NAAC Be-Accredited 'A'Grade o l.D. No.: PU/PNiA.S./009 (1945) M.Sc., M.Phil., Ph. D. o Best College Award - Savitribai Phule Pune University . JR. COLLEGE CODE: 11.003 Principal o Best Student Development Board Award - Savitribai Phule pune University . AISHE CODE : C-41477 Ref. No. Date: t 3ln 'f}tt Z oz1 DEPARTMENT OF BIOTECHNOLOGY F.Y" B. Sc. Biotechnology Admissions e0ll-22) NOTICE Merit prepared list eonsidering Physics, Chernistry and Biology (PCB) marks obtained at 12th Standard/ HSC Science or equivalenr examination" Revised Timelines and Instructions for filling Merit Apptication Form for admission to F" Y. B. Sc. Biotechnotogy e02t-22) S"No Date Step in Admission Time Details Procedure 1 a6l08l2a2t Online Merit 24x7 Refer Website Application Form available Complete the on-line Merit Application form filling 2 2010812021 Last date for Till 5:00 pm process using link: https://aec.vriddhionline.com submission of Merit . Application Form Click login- click Student Register Tab and proceed according to instructions. c Fill in all the details. o For Subjeets- Physics, Chemistry and Biology, if the marks given are not out of 100, please conveft and enter the marks out of 100. o Enter total PCB marks out of 300 o For HSC percentage, please ealculate o/o aggregate marks and enter. c Filling of false inforrnation will disqualiS, the student from the admission process. -

Annasaheb Kulkarni Department of Biodiversity
MES Abasaheb Garware College Karve road, Pune 46 Annasaheb Kulkarni Department of Biodiversity The first newsletter of Annasaheb Kulkarni Department of Biodiversity covers the activities from July 2019 to December 2019. This newsletter entails highlights pertaining to guest lectures, field work/study tours, research and outreach. 1. Shrirang Kulkarni from discussed about Crocodile culturing and conservation in the leather industry. This activity was designed to make the students aware of industry’s approach towards biodiversity based business opportunity. Biodiversity Department 2. Dr. Shonil Bhagwat from Open University, UK discussed about cultural factors that influence the ecological dynamics of the Western Ghats. This session was an open session for which the students of all the life sciences departments of the college, teachers from other colleges and people from various institutes in Pune attended. It was well received by all. Parent Teacher meeting for the parents of M.Sc. I was held on 28.09.2019. The rationale of the department, working and activities were explained to the parents. All the teaching staff was present for the PTM. Queries of the parents were also resolved during the meeting and we got a positive feedback from the parents on the activities of the department. Practical at Amboli Forest Activities at Waghapur region Biodiversity Department Scientific outreach . Talk by Dr. Dhanashree Paranjape at National Symposium on Avian Biology (7th to 10th December 2019). What’s in the spot?: Do peacocks select display territories based on proximity to important resources? . Poster presentation at ISEB1: Celebrating Ecology and Evolution in India (24th & 25th October 2019). To group or not to group: Does season and food provisioning affect group dynamics and parasite load in Indian Peafowl? . -

MES Abasaheb Garware College, Pune
M.E.S. Abasaheb Garware College, Pune – 411004 M.Sc.-I Chemistry Admission 2016-17 NO NEED TO SUBMIT HARD COPY OF APPLICATION FORM TO COLLEGE Application Fees Rs.200/- for Open Category and Rs.100/- for Other Category Admission Schedule Online application form will be available from 24 th June to 2 nd July 2016 (Please visit http://mesgarwarecollege.org/) Last date for online application is 2nd July 2016 . The merit list will be displayed on 7th July 2016 at 4.00 pm. Corrected list will be displayed on 9th July 2016 at 4.00 pm. Table admissions will be on 12 th July 2016 at 10.00 am in AV Hall (Selected candidate will have to bring all the original documents along with the necessary fee for admission to M.Sc. I) Admission process is as per the rules and regulations of Govt. of Maharashtra and Savitribai Phule Pune University. At the time of Table Admission, selected candidates will have to pay following fees. Analytical Chemistry Fee Structure Course Name Category Fees Analytical Chemistry SC and ST Rs.9700/- Analytical Chemistry For all other Candidate Rs.36,630/- Organic Chemistry Fee Structure Course Name Category Fees Organic Chemistry SC and ST Rs.5610/- Organic Chemistry EBC/PTC/STC/EX-SER Rs.7620/- Organic Chemistry GENERAL CATEGORY Rs.8790/- In addition Rs.2000/- is to be paid as instrumentation fee by Organic Chemistry Students. Payment Options: Online payment : Student can pay through Debit card, Credit card and net-banking after submission of their application form. D.D Payment : Student can select D.D option after submission of application form. -

M.Sc. I Biodiversity Coll
University of Pune M. Sc. Biodiversity – Monitoring and Utilization Credit & Semester System Syllabus Degree: Master of Science (M. Sc.) Part I Subject: Biodiversity Faculty: Science A.Y. : – 2014-15 General Information M. Sc. Biodiversity - Monitoring and Utilization (Credit & Semester System) is a two year postgraduate course, comprising four semesters and is a unique program conducted at Abasaheb Garware College, Pune; since 2003. The course was sanctioned under Innovative Programs Scheme of University Grants Commission. The curriculum gives holistic coverage to the extremely valuable field of Biodiversity. Biodiversity is the largest source of potential wealth for the country, which remains grossly under explored. One of the reasons for the under utilization is the dearth of trained manpower. The current generation of biologists is largely divided into field-oriented taxonomists and ecologists on the one hand and the lab oriented functional and molecular biologists on the other. This divide has become a limiting factor in the study of Biodiversity. The present program intends to bridge the gap by inculcating excellence in field and laboratory biology simultaneously. This capacity building exercise will help generating wealth through a prudent and sustainable use of the country’s bioresources. The course consists of four semesters; • The first year comprising two semesters is extensively field oriented and the second year is lab intensive. • The first semester is devoted to taxonomy and diversity of various life forms and emphasizes on basic techniques of exploration of diversity. • Second semester focuses on natural history and is supplemented adequately with quantitative techniques in biology and ecology. Human ecology component which forms the key component in shaping up of natural systems has also been included here. -
ABASAHEB GARWARE COLLEGE Karve Road, Deccan Gymkhana, Pune – 411 004
MAHARASHTRA EDUCATION SOCIETY'S (SINCE 1860) ABASAHEB GARWARE COLLEGE Karve Road, Deccan Gymkhana, Pune – 411 004. Maharashtra, India Ph: 020-41038200 E-mail : [email protected] www.garwarecollege.mespune.in Prof.(Dr.) P.B.Buchade . NAAC Re-Accredited 'A' Grade . I.D.No. PU/PN/A.S/009(1945) M.Sc., M.Phil, Ph.D. Best College Award - Savitribai Phule Pune University . JR.COLLEGE CODE : 11.11.003 Principal . Best Student Development Board Award - Savitribai Phule Pune University . AISHE CODE : C-41477 . PUN CODE : CAAP 010040 Date : 09/08/2021 Admission to First Year B.A. (2021-22) On-line Admission Process The admission process will start from 9/8/2021. Admission forms will be available on the college website https://garwarecollege.mespune.in or https://agc.vriddhionline.com Arts faculty students should not fill the merit forms. All further notices regarding admissions will be displayed on college website Admission Process- All interested students should fill the admission form In the first round approval will be given to students who have secured more than 75% marks for General category and 65% marks for reserved category If seats are available, the Second round approval will be given to students who have secured more than 65% marks for General category and 55% marks for reserved category If seats are available, the Third round approval will be given to students who have secured more than 55% marks for General category and 45% marks for reserved category In the fourth round admissions will be open to all depending upon availability of seats. Approval will be given only to the completely filled forms after 18/08/2021. -

AGC Prospectus 2021-22
Maharashtra Education Society’s ABASAHEB GARAWARE COLLEGE Kare Road, Pune 411004 Website : http: / /garwarecollege.mespune.in E-mail : [email protected] Tel : 020-41038200 Afliated to Savitribai Phule Pune University NAAC Reaccredited ‘A’ Grade (3rd Cycle) College Best College Award - Savitribai Phule Pune University Best Student Development Bord Award -Savitribai Phule Pune University I.D. No: PU/PN/C/009 (1945) / JR. COLLEGE CODE: 11.11.003 AISHE CODE: C-41477-2018 PROSPECTUS 2021-22 College Committed to Excellence in Education Maharashtra Education Society’s ABASAHEB GARAWARE COLLEGE Kare Road, Pune 411004 Ofce Bearers Senior College Principal : Prof. (Dr.) P. B. Buchade Vice – Principals : Prof. (Dr.) Sunita Bhagwat (Science) Dr. P.D. Sonawane (Administration) Dr. Aparna Agashe (Arts) Dr. Manisha Bharambe (Non-Grant) Junior College Vice – Principal : Mrs. Smita Kulkarni Supervisor : Ms. Jayshree Bhave Ofce Admin Registrar : Mr. K.J. Sable Librarian : Prof. (Dr.) Vandana Shelar Director, Physical Education : Prof. (Dr.) Asha Bengle Ofce Contact Nos. Ofce Phone : 020-41038200 Hostel : 020-41038325 E-mail : [email protected] Website : http://garwarecollege.mespune.in Ofce Timings (Except on Holidays and First and Third Saturdays) Monday to Saturday : 10.00 am to 5.40 pm Lunch Time : 1.30 pm to 2.00 pm Cash Transaction : 10.30 am to 1.30 pm College Timing : 7.15 am to 6.10 pm Library Timing Reading Hall : 8.30 am to 5.00 pm Night Reading Hall : 8 am to 8 pm (Only prior to commencement of University Examination) Transaction of Books : 9.00 am to 5.00 pm Founder Members of Maharashtra Education Society Vaman Prabhakar Bhave Gr. -

ACKWO'^BDGSMBWTS Present Work Is a Result of Help Rendered to Me By
II) ACKWO’^BDGSMBWTS Present work Is a result of help rendered to me by many authorities, friends and colleagues and family members. It is my pleasant duty to record my sincere acknowledgement to all of them. It is the only occasion, where I can express my deep sense of gratitude to my guide Dr. V.D. 7artak, Scientist B-1 and Head of the Botany Department, MACS for his tireless efforts and cooperation during the entire period of my research work and compilation of the thesis. I am also indebted to Or. K.R. Surange, Director, MACS Research Institute and Prof. Q.B. Deodikar, Ex- Director and Emeritus Professor, for their unfailing interest in the subject, encouragement and proriding necessary laboratory facilities. Dr. S.H. Godbole, Deputy Director of the Institute, has shown keen interest in the subject, without which the work would hare not been completed. I am thankful to Dr. D.D. Wani, Head of the Botany Department, Abasaheb Garware College, Pune, who has encouraged me to Join the research field and also provided due time facilities for my research project. (V I have to acknowledge the authorities of (1) Bota nical Survey of India, Western Circle, Pune for herbarium consultation and (2) Meteorological Department, Government of India, Pune for rendering valuable information regarding climatic data. Justice 3.G. Patwardhan, Prof. D.G. Varadpande, Dr. M.S. Kumbhojkar and Mrs. V.3. Ghate deserve special mention for going through the manusceipt and for their critical comments and valuable suggestions in the correc tion of the draft. I am grateful to Dr. -

DEPARTMENT of BIOTECHNOLOGY ADMISSION NOTICE for M.Sc
M.E.S. ABASAHEB GARWARE COLLEGE Karve Road, Pune 411004 NAAC Accredited ‘A’ Grade. Best College Award, Savitribai Phule Pune University Website: www.mesgarwarecollege.org DEPARTMENT OF BIOTECHNOLOGY ADMISSION NOTICE FOR M.Sc BIOTECHNOLOGY 2015 - 16 1. Eligibility: Bachelor of Science in Biotechnology with minimum 55% marks for open category and 50% for reserved category. 2. No entrance test will be conducted for admissions to M.Sc. Biotechnology for the academic year 2015-2016. 3. Admission will be based on the merit list prepared by considering marks obtained at B.Sc Biotechnology examination (out of 1800 marks). 4. Forms will be available from 15 th May 2015 through ‘ e-pravesh ’ link on Abasaheb Garware College website (www.mesgarwarecollege.org ). 5. Completed application form along with T.Y.B.Sc marklist must be submitted online till 5 days after declaration of B.Sc Biotechnology results by SPPU. 6. Attested copy of T.Y.B.Sc. mark sheet should be submitted till 5 days after declaration of B.Sc Biotechnology results by SPPU. Form number should be mentioned on the top right hand corner of the mark sheet. 7. A student will not be considered for admission if he/she fails to submit the mark sheet in time. 8. Reservation norms will be followed as per Government of Maharashtra and University of Pune rules. 9. For updates and other details visit: www.mesgarwarecollege.org . 10. Processing fee as follows: For Open Category students: Rs. 200/- For Reserved Category students: Rs. 150/- Payment Options: Online payment: Student can pay through Debit card, Credit card and net- banking after submission of their application form. -

A S G F Or Th He S 201 0-15 E
Maharashtra Education Society’s AABASAAHEB GGARWAARE CCOOLLEGEE Karve Road, Pune 411004, Maharashtra State SELF‐STUDY REPORT For The ACADEMIC YEARS 2010‐15 RD (3 CYCLE OF NAAC ACCREDITATION) Submitted to: National Assessment and Accreditation Council ((NAAC) P. O. Box No. 1075, Nagarbhavi, Bengaluru 560072 October 2015 MES’S ABASAHEB GARWARE COLLEGE, PUNE CONTENTS • Preface • Steering Committee • Executive Summary and SWOC Analysis • Profile of the College and Annexures [I, IIa, IIb, IIIa, IIIb] • Evaluative Report: Criteria-wise • Criterion I Curricular Aspects • Criterion II Teaching - Learning and Evaluation • Criterion III Research, Consultancy and Extension • Criterion IV Infrastructure and Learning Resources • Criterion V Student Support and Progression • Criterion VI Governance, Leadership and Management • Criterion VII Innovations and Best Practices • Evaluative Report: Department-wise Faculty of Science • Biodiversity • Biotechnology • Botany • Chemistry • Computer Science • Electronic Science • Mathematics • Microbiology • Physics • Statistics • Zoology Faculty of Arts • Economics • English • Education • Geography • Hindi • History • Journalism and Mass Communication • Library Science • Logic and Philosophy • Marathi • Political Science • Psychology • Sociology • Post-accreditation Initiatives • Declaration by the Principal of the College • Mandatory Compliance for Assessment and Accreditation of HEIs Annexures • Annexure IV Affiliation of courses of the College by the University • Annexure V UGC 12th Plan for Development Assistance SELF STUDY REPORT [3RD CYCLE] CONTENTS MES’S ABASAHEB GARWARE COLLEGE, PUNE PREFACE Maharashtra Education Society’s Abasaheb Garware College is a college of repute nationally. Keeping in line with its motto “facta non verba”, the college has worked steadfastly, voluntarily and successfully in the NAAC accreditation process. This is the 3rd Cycle of accreditation that the college is preparing for. The Self-Study Report (SSR) is being presented for the period 2010 - 2015. -
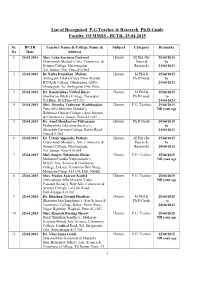
MMSS Ph.D Guide List 25.04.2015
List of Recognised P.G.Teacher & Research Ph.D.Guide Faculty Of MMSS - BUTR- 25.04.2015 Sr. BUTR Teacher Name & College Name & Subject Category Remarks No Date Address 1 25.04.2015 Shri. Lahu Kacharu Gaikwad History M.Phil (By 25/04/2015 Gramonnati Mandal’s Arts, Commerce, & Paper & to Science College, Narayangaon, Research) 24/04/2023 Tal. Junnar, Dist: Pune-410 504 2 25.04.2015 Dr.Natha Rambhau Mokate History M.Phil & 25/04/2015 Ambegaon Taluka Vidya Vikas Mandal Ph.D Guide to B.D.Kale College, Ghodegaon, At/Po- 24/04/2023 Ghodegaon, Ta: Ambegaon, Dist: Pune 3 25.04.2015 Dr. Ramkrishna Vitthal Dhere History M.Phil & 25/04/2015 Shankarrao Bhelke College, Nasarapur, Ph.D Guide to Tal.Bhor, Dist.Pune-412 213 24/04/2023 4 25.04.2015 Shri. Jitendra Yashwant Wadshingkar History P.G. Teacher 25/04/2015 Pune Jilha Shikshan Mandal’s, Till your age Baburaoji Gholap College, (Arts, Science & Commerce) Sangvi, Pune-411 027 5 25.04.2015 Dr. Amol Shankarrao Vidyasagar History Ph.D.Guide 25/04/2015 Maharashtra Education Society’s, to Abasaheb Garware College, Karve Road, 24/04/2023 Pune-411 004 6 25.04.2015 Dr. Uttam Appasahe Pathare History M.Phil (By 25/04/2015 Gramonnati Mandal’s, Arts, Commerce & Papers & to Science College, Narayangaon, Research) 24/04/2023 Tal: Junnar, Pune-410 504 7 25.04.2015 Shri. Sanjay Tulshiram Shelar History P.G. Teacher 25/04/2015 Mahatma Gandhi Vidyamandir’s, Till your age M.S.G. Arts, Science & Commerce College, Loknete Vynaktrao Hire Marg, Malegaon Camp, 423 105, Dist: Nashik 8 25.04.2015 Shri.