Adventures with Sugars
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Sequencing As a Way of Work
Edinburgh Research Explorer A new insight into Sanger’s development of sequencing Citation for published version: Garcia-Sancho, M 2010, 'A new insight into Sanger’s development of sequencing: From proteins to DNA, 1943-77', Journal of the History of Biology, vol. 43, no. 2, pp. 265-323. https://doi.org/10.1007/s10739-009- 9184-1 Digital Object Identifier (DOI): 10.1007/s10739-009-9184-1 Link: Link to publication record in Edinburgh Research Explorer Document Version: Peer reviewed version Published In: Journal of the History of Biology Publisher Rights Statement: © Garcia-Sancho, M. (2010). A new insight into Sanger’s development of sequencing: From proteins to DNA, 1943-77. Journal of the History of Biology, 43(2), 265-323. 10.1007/s10739-009-9184-1 General rights Copyright for the publications made accessible via the Edinburgh Research Explorer is retained by the author(s) and / or other copyright owners and it is a condition of accessing these publications that users recognise and abide by the legal requirements associated with these rights. Take down policy The University of Edinburgh has made every reasonable effort to ensure that Edinburgh Research Explorer content complies with UK legislation. If you believe that the public display of this file breaches copyright please contact [email protected] providing details, and we will remove access to the work immediately and investigate your claim. Download date: 28. Sep. 2021 THIS IS AN ADVANCED DRAFT OF A PUBLISHED PAPER. REFERENCES AND QUOTATIONS SHOULD ALWAYS BE MADE TO THE PUBLISHED VERION, WHICH CAN BE FOUND AT: García-Sancho M. -

Cambridge's 92 Nobel Prize Winners Part 2 - 1951 to 1974: from Crick and Watson to Dorothy Hodgkin
Cambridge's 92 Nobel Prize winners part 2 - 1951 to 1974: from Crick and Watson to Dorothy Hodgkin By Cambridge News | Posted: January 18, 2016 By Adam Care The News has been rounding up all of Cambridge's 92 Nobel Laureates, celebrating over 100 years of scientific and social innovation. ADVERTISING In this installment we move from 1951 to 1974, a period which saw a host of dramatic breakthroughs, in biology, atomic science, the discovery of pulsars and theories of global trade. It's also a period which saw The Eagle pub come to national prominence and the appearance of the first female name in Cambridge University's long Nobel history. The Gender Pay Gap Sale! Shop Online to get 13.9% off From 8 - 11 March, get 13.9% off 1,000s of items, it highlights the pay gap between men & women in the UK. Shop the Gender Pay Gap Sale – now. Promoted by Oxfam 1. 1951 Ernest Walton, Trinity College: Nobel Prize in Physics, for using accelerated particles to study atomic nuclei 2. 1951 John Cockcroft, St John's / Churchill Colleges: Nobel Prize in Physics, for using accelerated particles to study atomic nuclei Walton and Cockcroft shared the 1951 physics prize after they famously 'split the atom' in Cambridge 1932, ushering in the nuclear age with their particle accelerator, the Cockcroft-Walton generator. In later years Walton returned to his native Ireland, as a fellow of Trinity College Dublin, while in 1951 Cockcroft became the first master of Churchill College, where he died 16 years later. 3. 1952 Archer Martin, Peterhouse: Nobel Prize in Chemistry, for developing partition chromatography 4. -

RICHARD LAWRENCE MILLINGTON SYNGE BA, Phd(Cantab), Hondsc(Aberd), Hondsc(E.Anglia), Hon Dphil(Uppsala), FRS Nobel Laureate 1952
RICHARD LAWRENCE MILLINGTON SYNGE BA, PhD(Cantab), HonDSc(Aberd), HonDSc(E.Anglia), Hon DPhil(Uppsala), FRS Nobel Laureate 1952 R L M Synge was elected FRSE in 1963. He was born in West Kirby, Cheshire, on 28 October 1914, the son of Katherine (née Swan) and Lawrence Millington Synge, a Liverpool Stockbroker. The family was known to be living in Bridgenorth (Salop) in the early sixteenth century. At that time the name was Millington and there is a story that a member of the family from Millington Hall in Rostherne (Cheshire) sang so beautifully before King Henry VIII that he was told to take the name Synge. There have been various spellings of the name and in the nineteenth century the English branch settled on Sing which they retained until 1920 when both R M and L M Sing (Dick's uncle and father respectively) changed their names by deed poll to Synge. In the 19th and 20th centuries the Sing/Synge family played a considerable part in the life of Liverpool and Dick's father was High Sheriff of Cheshire in 1954. Dick was educated at Old Hall, a prep school in Wellington (Salop) and where he became renowned for his ability in Latin and Greek, subjects which he continued to study with such success at Winchester that in December 1931 when, just turned 17, he was awarded an Exhibition in Classics by Trinity College, Cambridge. His intention was to study science and Trinity allowed Dick to switch from classics to the Natural Sciences Tripos. To prepare for the change he returned to Winchester in January 1932 to study science for the next 18 months and was awarded the senior science prize for 1933. -

Pharmaceutical Compositions of Rifaximin Pharmazeutische Rifaximin-Zusammensetzungen Compositions Pharmaceutiques De Rifaximine
(19) TZZ Z__ T (11) EP 2 011 486 B2 (12) NEW EUROPEAN PATENT SPECIFICATION After opposition procedure (45) Date of publication and mention (51) Int Cl.: of the opposition decision: A61K 9/20 (2006.01) A61K 31/44 (2006.01) 12.08.2015 Bulletin 2015/33 (45) Mention of the grant of the patent: 23.05.2012 Bulletin 2012/21 (21) Application number: 08252198.0 (22) Date of filing: 26.06.2008 (54) Pharmaceutical compositions of rifaximin Pharmazeutische Rifaximin-Zusammensetzungen Compositions pharmaceutiques de rifaximine (84) Designated Contracting States: (56) References cited: AT BE BG CH CY CZ DE DK EE ES FI FR GB GR EP-A1- 0 616 808 EP-B1- 1 763 339 HR HU IE IS IT LI LT LU LV MC MT NL NO PL PT WO-A-2006/094737 WO-A2-2006/039022 RO SE SI SK TR US-A- 6 140 355 US-A1- 2005 101 598 (30) Priority: 06.07.2007 IN KO09682007 • DUPONT ET AL: "Treatment of Travelers’ 23.06.2008 EP 08252158 Diarrhea: Randomized Trial Comparing Rifaximin, Rifaximin Plus Loperamide, and (43) Date of publication of application: Loperamide Alone" CLINICAL 07.01.2009 Bulletin 2009/02 GASTROENTEROLOGY AND HEPATOLOGY, AMERICAN GASTROENTEROLOGICAL (60) Divisional application: ASSOCIATION, US, vol. 5, no. 4, 17 April 2007 11176043.5 / 2 420 226 (2007-04-17), pages 451-456, XP022029177 ISSN: 14186563.4 / 2 837 378 1542-3565 • ARYA ET AL: "Rifaximin-the promising anti- (73) Proprietor: Lupin Ltd. microbial for enteric infections" JOURNAL OF Mumbai, Maharashtra 400 098 (IN) INFECTION, ACADEMIC PRESS, LONDON, GB, vol. -

Ian Rae: “Two Croatian Chemists Who Were Awarded the Nobel Prize in Chemistry”
Croatian Studies Review 13 (2017) Ian Rae: “Two Croatian Chemists who were Awarded the Nobel Prize in Chemistry” Ian Rae School of Chemistry University of Melbourne [email protected] Abstract Two organic chemists of Croatian origin, Leopold Ružička and Vladimir Prelog, made significant contributions to natural product chemistry during the twentieth century. They received their university education and research training in Germany and Czechoslovakia, respectively. Both made their careers in Zürich, Switzerland, and both shared the Nobel Prize in Chemistry, in 1939 and 1975, respectively. In this article, I have set the details of their lives and achievements against the education and research climates in Europe and other regions, especially as they apply to the field of chemistry. Key words: Croatia, organic, chemistry, Nobel, Ružička, Prelog 31 Croatian Studies Review 13 (2017) Introduction1 In the twentieth century two organic chemists of Croatian origin were awarded the Nobel Prize in Chemistry. They were Lavoslav (Leopold) Ružička (1887-1976) and Vladimir Prelog (1906-1998), whose awards came in 1939 and 1975, respectively. Both were living and working in Switzerland at the time of the awards and it was in that country – specifically in the city of Zürich – that they performed the research that made them Nobel Laureates. To understand the careers of Ružička and Prelog, and of many other twentieth century organic chemists, we need to look back to the nineteenth century when German chemists were the leaders in this field of science. Two developments characterise this German hegemony: the introduction of the research degree of Doctor of Philosophy (PhD), and the close collaboration between organic chemists in industry and university. -

Sia: the Sugar Code of Life
Journal of General Surgery Open Access Full Text Article Research Article Sia: The Sugar Code of Life This article was published in the following Scient Open Access Journal: Journal of General Surgery Received November 05, 2017; Accepted November 27, 2017; Published December 04, 2017 Robert Skopec* Abstract Analyst-researcher, Dubnik, Slovakia, Europe Our cells are coated with sugar, and when it comes to cancer, that’s anything but sweet. In a recent talk at TEDxStanford, chemist Carolyn Bertozzi explained why. She studies sialic acid, a sugar that seems to deceive the immune system, allowing cancer cells to evade the body’s defenses. This work focuses on the complex, sugary structures surrounding human cells. That foliage-like coating, it turns out, can tell us a lot of our body – it even reveals a patient’s blood type. Sugar and carbohydrates are dangerous supporters of different types of cancer. Introduction As it can be seen from the information of the American Chemical Society (ACS), ideally, the immune system can figure out which cells are bad, attack them and protect the body from disease. In the case of cancer cells, though, a special sugary coating tricks the immune system into ignoring them. The sialic acid, a sugar that’s denser in cancer cells than in other cells. It seems deceive the immune system, allowing the cancer cells to evade the body’s defenses. Unnoticed and unchallenged, cancer cells are free to divideMonosaccharides and run wild inside - Sialic the acid/N-acetylneuraminicbody [1,2]. acid N-acetylneuraminic acid, also known as Sialic acid, is a key component of important amino sugars that mediate cellular communication. -
RSC Branding
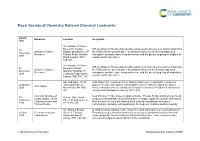
Royal Society of Chemistry National Chemical Landmarks Award Honouree Location Inscription Date The Institute of Cancer Research, Chester ICR scientists on this site and elsewhere pioneered numerous new cancer drugs from 10 Institute of Cancer Beatty Laboratories, 237 the 1950s until the present day – including the discovery of chemotherapy drug December Research Fulham Road, Chelsea carboplatin, prostate cancer drug abiraterone and the genetic targeting of olaparib for 2018 Road, London, SW3 ovarian and breast cancer. 6JB, UK The Institute of Cancer ICR scientists on this site and elsewhere pioneered numerous new cancer drugs from 10 Research, Royal Institute of Cancer the 1950s until the present day – including the discovery of chemotherapy drug December Marsden Hospital, 15 Research carboplatin, prostate cancer drug abiraterone and the genetic targeting of olaparib for 2018 Cotswold Road, Sutton, ovarian and breast cancer. London, SM2 5NG, UK Ape and Apple, 28-30 John Dalton Street was opened in 1846 by Manchester Corporation in honour of 26 October John Dalton Street, famous chemist, John Dalton, who in Manchester in 1803 developed the Atomic John Dalton 2016 Manchester, M2 6HQ, Theory which became the foundation of modern chemistry. President of Manchester UK Literary and Philosophical Society 1816-1844. Chemical structure of Near this site in 1903, James Colquhoun Irvine, Thomas Purdie and their team found 30 College Gate, North simple sugars, James a way to understand the chemical structure of simple sugars like glucose and lactose. September Street, St Andrews, Fife, Colquhoun Irvine and Over the next 18 years this allowed them to lay the foundations of modern 2016 KY16 9AJ, UK Thomas Purdie carbohydrate chemistry, with implications for medicine, nutrition and biochemistry. -

Michael Rossmann
• • Purdue College of Science | Spring 2017 MICHAEL ROSSMANN: HIS PATH TO PURDUE & DECADES OF DISCOVERY @PurdueScience INSIDE: ARTIFICIAL INTELLIGENCE :: ALUMNUS’S NEXT NASA MISSION Dr.JD For more than 50 years, Michael Rossmann, the Hanley Distinguished Professor of Biological Sciences, walked to his labs in Lilly Hall and Hockmeyer Hall of Structural Biology from his West Lafayette home. It was a leisurely 30 -minute stroll, at just over a mile to the southern tip of the Purdue campus. However, if one adds up all of his trips, he has traveled a distance equal to a round trip to Rio de Janeiro, Brazil, and back again to Rio. During those walks down Grant Street — past the blocks of homes built in the early 20th century as Purdue University grew and through an expanding campus — Rossmann’s mind stormed with ideas. Ideas mulled on these walks have led to monumental discoveries in the field of structural biology. Rossmann’s discoveries have helped doctors under - stand, treat and even cure infections from alpha viruses, coxsackievirus B3, flaviviruses like dengue and Zika, and even the rhinovirus that causes the common cold. His latest work has been a collaborative effort with Richard Kuhn, professor of biological sciences and director of the Purdue Institute for Inflammation, Immunology and Infectious Disease, to study Zika virus. The virus has received widespread attention because of an increase in microcephaly — a birth defect that causes brain damage and an abnormally small head in babies born to some mothers infected during pregnancy — and reported transmission of the mosquito -borne virus in 33 countries. -

Pharmaceutical Compositions for Gastrointestinal Drug Delivery
(19) TZZ Z¥_T (11) EP 2 942 053 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 11.11.2015 Bulletin 2015/46 A61K 9/16 (2006.01) A61K 9/20 (2006.01) A61K 31/437 (2006.01) A61K 31/606 (2006.01) (2006.01) (2006.01) (21) Application number: 15156572.8 A61K 31/635 A61K 38/14 A61K 31/655 (2006.01) A61K 9/00 (2006.01) (2006.01) (22) Date of filing: 26.06.2008 A61K 9/24 (84) Designated Contracting States: • Kulkarni, Rajesh AT BE BG CH CY CZ DE DK EE ES FI FR GB GR 411 042 Pune (IN) HR HU IE IS IT LI LT LU LV MC MT NL NO PL PT • Kulkarni, Shirishkumar RO SE SI SK TR 411 042 Pune (IN) (30) Priority: 06.07.2007 IN KO09692007 (74) Representative: Hoffmann Eitle 24.06.2008 EP 08252164 Patent- und Rechtsanwälte PartmbB Arabellastraße 30 (62) Document number(s) of the earlier application(s) in 81925 München (DE) accordance with Art. 76 EPC: 08252197.2 / 2 011 487 Remarks: •This application was filed on 25-02-2015 as a (71) Applicant: Lupin Limited divisional application to the application mentioned Mumbai, Maharashtra 400 098 (IN) under INID code 62. •Claims filed after the date of filing of the application (72) Inventors: (Rule 68(4) EPC). • Jahagirdar, Harshal Anil 411 042 Pune (IN) (54) Pharmaceutical compositions for gastrointestinal drug delivery (57) A pharmaceutical composition, which compris- increase the residence time of the said pharmaceutical es a therapeutically effective amount of active principle composition and/or active principle (s) in the gastrointes- (s) or a pharmaceutically acceptable salt or enantiomer tinal tract. -

Los Premios Nobel De Química
Los premios Nobel de Química MATERIAL RECOPILADO POR: DULCE MARÍA DE ANDRÉS CABRERIZO Los premios Nobel de Química El campo de la Química que más premios ha recibido es el de la Quí- mica Orgánica. Frederick Sanger es el único laurea- do que ganó el premio en dos oca- siones, en 1958 y 1980. Otros dos también ganaron premios Nobel en otros campos: Marie Curie (física en El Premio Nobel de Química es entregado anual- 1903, química en 1911) y Linus Carl mente por la Academia Sueca a científicos que so- bresalen por sus contribuciones en el campo de la Pauling (química en 1954, paz en Física. 1962). Seis mujeres han ganado el Es uno de los cinco premios Nobel establecidos en premio: Marie Curie, Irène Joliot- el testamento de Alfred Nobel, en 1895, y que son dados a todos aquellos individuos que realizan Curie (1935), Dorothy Crowfoot Ho- contribuciones notables en la Química, la Física, la dgkin (1964), Ada Yonath (2009) y Literatura, la Paz y la Fisiología o Medicina. Emmanuelle Charpentier y Jennifer Según el testamento de Nobel, este reconocimien- to es administrado directamente por la Fundación Doudna (2020) Nobel y concedido por un comité conformado por Ha habido ocho años en los que no cinco miembros que son elegidos por la Real Aca- demia Sueca de las Ciencias. se entregó el premio Nobel de Quí- El primer Premio Nobel de Química fue otorgado mica, en algunas ocasiones por de- en 1901 al holandés Jacobus Henricus van't Hoff. clararse desierto y en otras por la Cada destinatario recibe una medalla, un diploma y situación de guerra mundial y el exi- un premio económico que ha variado a lo largo de los años. -

Sialic Acid (N-Acetylneuraminic Acid)
Kim et al., J Glycomics Lipidomics 2014, 4:1 DOI: 10.4172/2153-0637.1000e116 Journal of Glycomics & Lipidomics Editorial Open Access Sialic Acid (N-Acetylneuraminic Acid) as the Functional Molecule for Differentiation between Animal and Plant Kingdom Cheorl-Ho Kim* Molecular and Cellular Glycobiology Unit, Department of Biological Sciences, College of Science, Sungkyunkwan University, Chunchun-Dong 300, Jangan-Gu, Suwon City, Kyunggi-Do 440-746, South Korea Keywords: Sialic acid; N-Acetylneuraminic acid; Echinoderms; Biological functionof Sialic Acids Animal-specific characters; Evolution The sialic acids or Neu5Ac are a group of 9-carbon monosacchride Organisms synthesize saccharides for carbohydrates, amino and synthesized in animals (Figure 2) [1]. Sialic acid-containing acids for proteins, fatty acid for lipids and nucleotides for nucleic glycoconjugates are initially synthesized from the deuterostome acids for the basic molecules. Recently, carbohydrates have been lineage of the echinoderms such as starfish and sea urchin up to the recognized as the 3rd life chain molecule in eukaryotic cells. One higher mammals. The echinoderms emerged some 500 million years of the biggest differences between the plant and animal kingdom ago. In insects and gastropod, the content of sialic acids are extremely would be the existence of the 9-carbon monosaccharide, sialic low [4-6] and protostome animals do not produce the sialic acids as forms of glycoconjugates [7]. In sialic acid-producing organisms, they acid or N-acetylneuraminic acids (Neu5Ac) (Figure 1). Even some occur as terminal residues in the glycoconjugates of cell surface and enterobacterial species produce the sialic acids, although their are components of glycoproteins, glycolipid such as ad gangliosides origins are postulated to be probably derived from the bacteria-host and glycosaminoglycan ubiquitously present in mammals and lower interactions during long evolution. -

A Nobel Synthesis
MILESTONES IN CHEMISTRY Ian Grayson A nobel synthesis IAN GRAYSON Evonik Degussa GmbH, Rodenbacher Chaussee 4, Hanau-Wolfgang, 63457, Germany he first Nobel Prize for chemistry was because it is a scientific challenge, as he awarded in 1901 (to Jacobus van’t Hoff). described in his Nobel lecture: “The synthesis T Up to 2010, the chemistry prize has been of brazilin would have no industrial value; awarded 102 times, to 160 laureates, of whom its biological importance is problematical, only four have been women (1). The most but it is worth while to attempt it for the prominent area for awarding the Nobel Prize sufficient reason that we have no idea how for chemistry has been in organic chemistry, in to accomplish the task” (4). which the Nobel committee includes natural Continuing the list of Nobel Laureates in products, synthesis, catalysis, and polymers. organic synthesis we arrive next at R. B. This amounts to 24 of the prizes. Reading the Woodward. Considered by many the greatest achievements of the earlier organic chemists organic chemist of the 20th century, he who were recipients of the prize, we see that devised syntheses of numerous natural they were drawn to synthesis by the structural Alfred Nobel, 1833-1896 products, including lysergic acid, quinine, analysis and characterisation of natural cortisone and strychnine (Figure 1). 6 compounds. In order to prove the structure conclusively, some In collaboration with Albert Eschenmoser, he achieved the synthesis, even if only a partial synthesis, had to be attempted. It is synthesis of vitamin B12, a mammoth task involving nearly 100 impressive to read of some of the structures which were deduced students and post-docs over many years.