Public and Private Sector Contributions to the Research & Development of the Most Transformational Drugs of the Last 25 Y
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Dovato, INN-Dolutegravir, Lamivudine
26 April 2019 EMA/267082/2019 Committee for Medicinal Products for Human Use (CHMP) Assessment report Dovato International non-proprietary name: dolutegravir / lamivudine Procedure No. EMEA/H/C/004909/0000 Note Assessment report as adopted by the CHMP with all information of a commercially confidential nature deleted. Downloaded from wizmed.com Official address Domenico Scarlattilaan 6 ● 1083 HS Amsterdam ● The Netherlands Address for visits and deliveries Refer to www.ema.europa.eu/how-to-find-us Send us a question Go to www.ema.europa.eu/contact Telephone +31 (0)88 781 6000 An agency of the European Union © European Medicines Agency, 2019. Reproduction is authorised provided the source is acknowledged. Table of contents 1. Background information on the procedure .............................................. 6 1.1. Submission of the dossier ...................................................................................... 6 1.2. Steps taken for the assessment of the product ......................................................... 7 2. Scientific discussion ................................................................................ 8 2.1. Problem statement ............................................................................................... 8 2.1.1. Disease or condition ........................................................................................... 8 2.1.2. Epidemiology .................................................................................................... 9 2.1.3. Biologic features ............................................................................................... -

Second Quarter 2017
Press release Second quarter 2017 Issued: Wednesday, 26 July 2017, London U.K. GSK delivers further progress in Q2 and sets out new priorities for the Group Q2 sales of £7.3 billion, +12% AER, +3% CER Total loss per share of 3.7p, +59% AER, +29% CER; Adjusted EPS of 27.2p, +12% AER, -2% CER Financial highlights • Pharmaceutical sales, £4.4 billion, +12% AER, +3% CER, Vaccines sales, £1.1 billion, +16% AER, +5% CER, Consumer Healthcare sales, £1.9 billion,+10% AER, flat at CER • Group operating margin 28.5%; Pharmaceuticals 33.6%; Vaccines 33.7%; Consumer 17.7% • Total Q2 loss per share of 3.7p reflecting charges resulting from increases in the valuation of Consumer and HIV businesses and new portfolio choices • Updated 2017 guidance: Adjusted EPS growth now expected to be 3% to 5% CER reflecting impact of Priority Review Voucher • H1 Free Cash Flow £0.4 billion (H1 2016: £0.1 billion) • 19p dividend declared for Q2; continue to expect 80p for FY 2017 Product and pipeline highlights • New product sales of £1.7 billion, +62% AER, +47% CER • HIV two drug regimen (dolutegravir and rilpivirine) filed for approval in US and EU • Shingrix filed for approval in Japan • FDA approval received for subcutaneous Benlysta for treatment of SLE New business priorities to 2020 • New priorities to strengthen innovation, improve performance and build trust • Pharmaceutical R&D pipeline reviewed with target over time to allocate 80% of capital to priority assets in two current (Respiratory and HIV/infectious diseases) and two potential (Oncology and Immuno-inflammation) -

Dovato, INN-Dolutegravir, Lamivudine
26 April 2019 EMA/267082/2019 Committee for Medicinal Products for Human Use (CHMP) Assessment report Dovato International non-proprietary name: dolutegravir / lamivudine Procedure No. EMEA/H/C/004909/0000 Note Assessment report as adopted by the CHMP with all information of a commercially confidential nature deleted. Official address Domenico Scarlattilaan 6 ● 1083 HS Amsterdam ● The Netherlands Address for visits and deliveries Refer to www.ema.europa.eu/how-to-find-us Send us a question Go to www.ema.europa.eu/contact Telephone +31 (0)88 781 6000 An agency of the European Union © European Medicines Agency, 2019. Reproduction is authorised provided the source is acknowledged. Table of contents 1. Background information on the procedure .............................................. 6 1.1. Submission of the dossier ...................................................................................... 6 1.2. Steps taken for the assessment of the product ......................................................... 7 2. Scientific discussion ................................................................................ 8 2.1. Problem statement ............................................................................................... 8 2.1.1. Disease or condition ........................................................................................... 8 2.1.2. Epidemiology .................................................................................................... 9 2.1.3. Biologic features ............................................................................................... -
Analyst Report
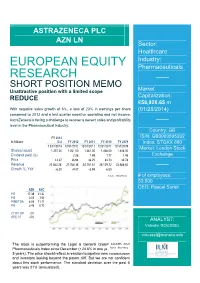
ASTRAZENECA PLC AZN LN Sector: Healthcare Industry: EUROPEAN EQUITY Pharmaceuticals RESEARCH I SHORT POSITION MEMO Market Unattractive position with a limited scope REDUCE Capitalization: €58,920.65 m With negative sales growth of 6 %, a loss of 23% in earnings per share (01/28/2014) compared to 2012 and a last quarter negative operating and net income, AstraZeneca is facing a challenge to recover a decent sales and profitability level in the Pharmaceutical industry. Country: GB FY 2013 ISIN: GB0009895292 In Millions Est FY 2012 FY 2011 FY 2010 FY 2009 Index: STOXX 600 12/31/2013 12/31/2012 12/31/2011 12/31/2010 12/31/2009 Market: London Stock Shares issued 1,257.00 1,261.00 1,361.00 1,438.00 1,448.00 Dividend yield (%) Exchange 2.26 1.99 1.77 1.48 Price 43.07 36.94 36.25 34.73 32.78 Revenue 20,462.28 21,768.38 24,151.51 25,129.22 23,588.60 oji Growth %, YoY -6.00 -9.87 -3.89 6.53 Source : Bloomberg # of employees: 53,500 CEO: Pascal Soriot AZN AVG P/E 17.38 21.32 P/S 3.05 3.56 P/EBITDA 8.04 11.11 P/B 3.45 5.72 2Y RV GR -2% OPE CF -5% ANALYST: Valentin ROUSSEL [email protected] The stock is outperforming the Legal & General Global Health and Pharmaceuticals Index since December (+24.6% in one year,Source +17.8% : Bloomberg in 5 years). The price should reflect a reaction to pipeline new introductions and investors looking beyond the patent cliff. -

Product Monograph for CELSENTRI
PRODUCT MONOGRAPH PrCELSENTRI maraviroc Tablets 150 and 300 mg CCR5 antagonist ViiV Healthcare ULC 245, boulevard Armand-Frappier Laval, Quebec H7V 4A7 Date of Revision: July 05, 2019 Submission Control No: 226222 © 2019 ViiV Healthcare group of companies or its licensor. Trademarks are owned by or licensed to the ViiV Healthcare group of companies. Page 1 of 60 Table of Contents PART I: HEALTH PROFESSIONAL INFORMATION.........................................................3 SUMMARY PRODUCT INFORMATION ........................................................................3 INDICATIONS AND CLINICAL USE..............................................................................3 CONTRAINDICATIONS ...................................................................................................3 WARNINGS AND PRECAUTIONS..................................................................................4 ADVERSE REACTIONS....................................................................................................9 DRUG INTERACTIONS ..................................................................................................19 DOSAGE AND ADMINISTRATION..............................................................................28 OVERDOSAGE ................................................................................................................31 ACTION AND CLINICAL PHARMACOLOGY ............................................................31 STORAGE AND STABILITY..........................................................................................36 -

Product Monograph for COMBIVIR
PRODUCT MONOGRAPH PrCOMBIVIR® lamivudine and zidovudine 150 mg of lamivudine and 300 mg zidovudine tablets Antiretroviral Agent ViiV Healthcare ULC 245 Boulevard Armand-Frappier Laval, Quebec H7V 4A7 Date of Revision: June 06, 2019 Submission Control No: 226229 © 2019 ViiV Healthcare ULC. All Rights Reserved. COMBIVIR is a registered trademark of the ViiV Healthcare group of companies. Page 1 of 49 Table of Contents PART I: HEALTH PROFESSIONAL INFORMATION .........................................................3 SUMMARY PRODUCT INFORMATION ........................................................................3 INDICATIONS AND CLINICAL USE ..............................................................................3 CONTRAINDICATIONS ...................................................................................................3 WARNINGS AND PRECAUTIONS ..................................................................................4 ADVERSE REACTIONS ..................................................................................................10 DRUG INTERACTIONS ..................................................................................................15 DOSAGE AND ADMINISTRATION ..............................................................................20 OVERDOSAGE ................................................................................................................20 ACTION AND CLINICAL PHARMACOLOGY ............................................................21 STORAGE AND STABILITY ..........................................................................................23 -

Affymetrix and the "DNA Chip" Revolution
taken pride in placing in the top 10 among the The Evolution of Astra best companies to work for in a number of ∗ magazine surveys, from Fortune to Working Merck Inc. in 1998 Mother. Merck & Co. sales grew from $9.6 billion in 1992 to $23 billion by the end of "We plan to revolutionize the pharmaceutical industry by 19972, topping the world drug sales league in being the best at linking patients and products.” Wayne Yetter, President of Astra Merck Inc. both years. In fact, Merck has seen its global market share jump one-third in the 1992-1998 period to edge Glaxo Wellcome in 1997 with a Section I. Background 3 4.6% world market share. In contrast to the In 1982, Merck & Co. and Astra AB signed an strategy of other pharmaceutical companies such agreement by which products resulting from as Glaxo, this growth was largely fueled by Astra’s research pipeline would be taken through internal R&D efforts as opposed to mergers and clinical trials and registration, to be ultimately acquisitions. marketed and sold by Merck in the United States. The initial terms of the contract specified Another major strategic move made to enhance that Astra’s products would be transferred to its competitive position was the acquisition of Merck’s development and marketing Medco Containment Services Inc. in 1993 organizations, with Astra receiving royalties on (renamed Merck-Medco Managed Care). the sales of the Astra drugs.1 Merck-Medco provides pharmaceutical benefit services in the United States to control The initial cooperation and relationship between prescription drug benefits cost. -

Building Innovation, Performance and Trust
Building Innovation, Performance and Trust Emma Walmsley, CEO Cautionary statement regarding forward-looking statements This presentation may contain forward-looking statements. Forward-looking statements give the Group’s current expectations or forecasts of future events. An investor can identify these statements by the fact that they do not relate strictly to historical or current facts. They use words such as ‘anticipate’, ‘estimate’, ‘expect’, ‘intend’, ‘will’, ‘project’, ‘plan’, ‘believe’, ‘target’ and other words and terms of similar meaning in connection with any discussion of future operating or financial performance. In particular, these include statements relating to future actions, prospective products or product approvals, future performance or results of current and anticipated products, sales efforts, expenses, the outcome of contingencies such as legal proceedings, and financial results. Other than in accordance with its legal or regulatory obligations (including under the Market Abuse Regulations, UK Listing Rules and the Disclosure and Transparency Rules of the Financial Conduct Authority), the Group undertakes no obligation to update any forward-looking statements, whether as a result of new information, future events or otherwise. Investors should, however, consult any additional disclosures that the Group may make in any documents which it publishes and/or files with the US Securities and Exchange Commission (SEC). All investors, wherever located, should take note of these disclosures. Accordingly, no assurance can be given that any particular expectation will be met and investors are cautioned not to place undue reliance on the forward-looking statements. Forward-looking statements are subject to assumptions, inherent risks and uncertainties, many of which relate to factors that are beyond the Group’s control or precise estimate. -

SHIRE PLC (Exact Name of Registrant As Specified in Its Charter)
UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM 10-K [X] ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the fiscal year ended December 31, 2009 [ ] TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 Commission file number 0-29630 SHIRE PLC (Exact name of registrant as specified in its charter) Jersey (Channel Islands) 98-0601486 (State or other jurisdiction of incorporation or (I.R.S. Employer Identification No.) organization) 5 Riverwalk, Citywest Business Campus, Dublin +353 1 429 7700 24, Republic of Ireland (Address of principal executive offices and zip code) (Registrant’s telephone number, including area code) Securities registered pursuant to Section 12(b) of the Act: Title of each class Name of exchange on which registered American Depositary Shares, each representing three NASDAQ Global Select Market Ordinary Shares 5 pence par value per share Securities registered pursuant to Section 12(g) of the Act: None (Title of class) 1 Indicate by check mark whether the Registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act Yes [X] No [ ] Indicate by check mark if the Registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act Yes [ ] No [X] Indicate by check mark whether the Registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. -

Pristine English Pm Tivicay 25-Jan-2021
PRODUCT MONOGRAPH PrTIVICAY Dolutegravir tablets Tablets, 10, 25 and 50 mg dolutegravir (as dolutegravir sodium) Dolutegravir dispersible tablets Dispersible tablets, 5 mg dolutegravir (as dolutegravir sodium) Antiretroviral Agent ViiV Healthcare ULC 245, boulevard Armand-Frappier Laval, Quebec H7V 4A7 Date of Revision: January 25, 2021 Submission Control No: 235583 © 2021 ViiV Healthcare group of companies or its licensor Trademarks are owned by or licensed to the ViiV Healthcare group of companies. Page 1 of 59 TABLE OF CONTENTS PAGE PART I: HEALTH PROFESSIONAL INFORMATION ........................................................ 3 SUMMARY PRODUCT INFORMATION ................................................................... 3 INDICATIONS AND CLINICAL USE ........................................................................ 3 CONTRAINDICATIONS ........................................................................................... 4 WARNINGS AND PRECAUTIONS............................................................................ 4 ADVERSE REACTIONS............................................................................................ 8 DRUG INTERACTIONS .......................................................................................... 16 DOSAGE AND ADMINISTRATION ........................................................................ 21 OVERDOSAGE....................................................................................................... 24 ACTION AND CLINICAL PHARMACOLOGY........................................................ -

Astrazeneca Plc
6/15/2020 AstraZeneca - Wikipedia AstraZeneca AstraZeneca plc[3] is a British-Swedish multinational pharmaceutical and biopharmaceutical company with its global AstraZeneca plc headquarters in Cambridge, England.[4] Its R&D is concentrated in Cambridge, Gaithersburg, Maryland, and Mölndal in Sweden.[5] AstraZeneca has a portfolio of products for major disease areas including cancer, cardiovascular, gastrointestinal, infection, Type Public limited neuroscience, respiratory and inflammation.[6] company Traded as LSE: AZN (https:// The company was founded in 1999 through the merger of the www.londonstocke [7][8] Swedish Astra AB and the British Zeneca Group (itself formed xchange.com/exch by the demerger of the pharmaceutical operations of Imperial ange/searchengin Chemical Industries in 1993). Since the merger it has been among e/search.html?lang the world's largest pharmaceutical companies and has made =en&x=0&y=0&q= numerous corporate acquisitions, including Cambridge Antibody AZN) Technology (in 2006), MedImmune (in 2007), Spirogen (in 2013) NYSE: AZN (http and Definiens (by MedImmune in 2014). s://www.nyse.com/ quote/XNYS:AZN) AstraZeneca has a primary listing on the London Stock Exchange Nasdaq and is a constituent of the FTSE 100 Index. It has secondary listings Stockholm: AZN (ht on the New York Stock Exchange and the OMX exchange. tp://www.nasdaqom xnordic.com/aktier/ microsite?language Contents Id=1&Instrument=S SE3524) History FTSE 100 2000–06 Component 2007–12: The patent cliff and subsequent acquisitions ISIN GB0009895292 2013 -

How to Sustain Healthcare Transformations
Voices on transformation A marathon, not a sprint: How to sustain healthcare transformations Voices on transformation is written by experts and practitioners in McKinsey & Company’s Pharmaceuticals and Medical Products Practice. To send comments or request copies of this publication, please email us at [email protected] Editors: Gayane Gyurjyan, Ioana Parsons, Shail Thaker, Jill Willder, Carla Zwaanstra Artwork and design: Afitha de Rijk-Voeten Portrait illustrations: Allan Burch Special thanks to: Lucia Darino, Martin Dewhurst, Claudio Feser, Vincent Forlenza, Jane Griffiths, Judith Hazlewood, Nadine Mansour, Angelika Reich, Pascal Soriot, David Speiser, Kirsten Westhues, André Wyss Copyright © 2016 McKinsey & Company. All rights reserved. This publication is not intended to be used as the basis for trading in the shares of any company or for undertaking any other complex or significant financial transaction without consulting appropriate professional advisers. No part of this publication may be copied or redistributed in any form without the prior written consent of McKinsey & Company. 2 PMP Voices on transformation <Chaper title> Contents Introduction 4 Cracking the code: How successful pharma leaders 7 manage transformations A health check for pharma: Overcoming change fatigue 15 in the pharmaceutical industry Putting science at the heart of renewed purpose 26 An interview with Pascal Soriot, AstraZeneca Transforming a medical devices company into a solutions provider 34 An interview with Vincent Forlenza, Becton Dickinson Refocusing the business around patient outcomes 42 An interview with Jane Griffiths, Janssen EMEA NBS: Creating value across Novartis 48 An interview with André Wyss, Novartis Voices on digital: How pharma can win in a digital world 57 About the authors 66 3 Introduction Over the past decade, the pharmaceutical industry has been struggling to keep up with rapid and dramatic changes in the external environment.