Acute Kidney Injury Mark D
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Impact of Urolithiasis and Hydronephrosis on Acute Kidney Injury in Patients with Urinary Tract Infection
bioRxiv preprint doi: https://doi.org/10.1101/2020.07.13.200337; this version posted July 13, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. Impact of urolithiasis and hydronephrosis on acute kidney injury in patients with urinary tract infection Short title: Impact of urolithiasis and hydronephrosis on AKI in UTI Chih-Yen Hsiao1,2, Tsung-Hsien Chen1, Yi-Chien Lee3,4, Ming-Cheng Wang5,* 1Division of Nephrology, Department of Internal Medicine, Ditmanson Medical Foundation Chia-Yi Christian Hospital, Chia-Yi, Taiwan 2Department of Hospital and Health Care Administration, Chia Nan University of Pharmacy and Science, Tainan, Taiwan 3Department of Internal Medicine, Fu Jen Catholic University Hospital, Fu Jen Catholic University, New Taipei, Taiwan 4School of Medicine, College of Medicine, Fu Jen Catholic University, New Taipei, Taiwan 5Division of Nephrology, Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan *[email protected] 1 bioRxiv preprint doi: https://doi.org/10.1101/2020.07.13.200337; this version posted July 13, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. Abstract Background: Urolithiasis is a common cause of urinary tract obstruction and urinary tract infection (UTI). This study aimed to identify whether urolithiasis with or without hydronephrosis has an impact on acute kidney injury (AKI) in patients with UTI. -

Uremic Toxins Affect Erythropoiesis During the Course of Chronic
cells Review Uremic Toxins Affect Erythropoiesis during the Course of Chronic Kidney Disease: A Review Eya Hamza 1, Laurent Metzinger 1,* and Valérie Metzinger-Le Meuth 1,2 1 HEMATIM UR 4666, C.U.R.S, Université de Picardie Jules Verne, CEDEX 1, 80025 Amiens, France; [email protected] (E.H.); [email protected] (V.M.-L.M.) 2 INSERM UMRS 1148, Laboratory for Vascular Translational Science (LVTS), UFR SMBH, Université Sorbonne Paris Nord, CEDEX, 93017 Bobigny, France * Correspondence: [email protected]; Tel.: +33-2282-5356 Received: 17 July 2020; Accepted: 4 September 2020; Published: 6 September 2020 Abstract: Chronic kidney disease (CKD) is a global health problem characterized by progressive kidney failure due to uremic toxicity and the complications that arise from it. Anemia consecutive to CKD is one of its most common complications affecting nearly all patients with end-stage renal disease. Anemia is a potential cause of cardiovascular disease, faster deterioration of renal failure and mortality. Erythropoietin (produced by the kidney) and iron (provided from recycled senescent red cells) deficiencies are the main reasons that contribute to CKD-associated anemia. Indeed, accumulation of uremic toxins in blood impairs erythropoietin synthesis, compromising the growth and differentiation of red blood cells in the bone marrow, leading to a subsequent impairment of erythropoiesis. In this review, we mainly focus on the most representative uremic toxins and their effects on the molecular mechanisms underlying anemia of CKD that have been studied so far. Understanding molecular mechanisms leading to anemia due to uremic toxins could lead to the development of new treatments that will specifically target the pathophysiologic processes of anemia consecutive to CKD, such as the newly marketed erythropoiesis-stimulating agents. -

The Links Between Microbiome and Uremic Toxins in Acute Kidney Injury: Beyond Gut Feeling—A Systematic Review
toxins Article The Links between Microbiome and Uremic Toxins in Acute Kidney Injury: Beyond Gut Feeling—A Systematic Review Alicja Rydzewska-Rosołowska 1,* , Natalia Sroka 1, Katarzyna Kakareko 1, Mariusz Rosołowski 2 , Edyta Zbroch 1 and Tomasz Hryszko 1 1 2nd Department of Nephrology and Hypertension with Dialysis Unit, Medical University of Białystok, 15-276 Białystok, Poland; [email protected] (N.S.); [email protected] (K.K.); [email protected] (E.Z.); [email protected] (T.H.) 2 Department of Gastroenterology and Internal Medicine, Medical University of Białystok, 15-276 Białystok, Poland; [email protected] * Correspondence: [email protected] Received: 30 October 2020; Accepted: 9 December 2020; Published: 11 December 2020 Abstract: The last years have brought an abundance of data on the existence of a gut-kidney axis and the importance of microbiome in kidney injury. Data on kidney-gut crosstalk suggest the possibility that microbiota alter renal inflammation; we therefore aimed to answer questions about the role of microbiome and gut-derived toxins in acute kidney injury. PubMed and Cochrane Library were searched from inception to October 10, 2020 for relevant studies with an additional search performed on ClinicalTrials.gov. We identified 33 eligible articles and one ongoing trial (21 original studies and 12 reviews/commentaries), which were included in this systematic review. Experimental studies prove the existence of a kidney-gut axis, focusing on the role of gut-derived uremic toxins and providing concepts that modification of the microbiota composition may result in better AKI outcomes. Small interventional studies in animal models and in humans show promising results, therefore, microbiome-targeted therapy for AKI treatment might be a promising possibility. -

Full Text (PDF)
www.jasn.org EDITORIALS 5. Grimm PR, Coleman R, Delpire E, Welling PA: Constitutively active Cachexia with muscle wasting is prevalent and closely associated SPAK causes hyperkalemia by activating NCC and remodeling distal with mortality and morbidity in patients with CKD.1 The patho- – tubules. JAmSocNephrol28: 2597 2606, 2017 physiology of muscle wasting in CKD is complex. Inadequate 6. Moriguchi T, Urushiyama S, Hisamoto N, Iemura S, Uchida S, Natsume T, Matsumoto K, Shibuya H: WNK1 regulates phosphorylation of cation- nutritional intake, physical inactivity from muscle weakness, sys- chloride-coupled cotransporters via the STE20-related kinases, SPAK temic inflammation and aberrant signaling of neuropeptides have and OSR1. J Biol Chem 280: 42685–42693, 2005 been implicated.2 To date, there is no effective therapy. Exercise 7. YangSS,MorimotoT,RaiT,ChigaM,SoharaE,OhnoM,UchidaK,LinSH, training has well documented health benefits, including mainte- Moriguchi T, Shibuya H, Kondo Y, SasakiS,UchidaS:Molecularpatho- nance of muscle mass as well as increased physical performance genesis of pseudohypoaldosteronism type II: Generation and analysis of a fi Wnk4(D561A/1) knockin mouse model. Cell Metab 5: 331–344, 2007 resulting from changes in muscle ber phenotype leading to in- 8. Lalioti MD, Zhang J, Volkman HM, Kahle KT, Hoffmann KE, Toka HR, Nelson- creased mitochondrial biogenesis. The molecular signaling mech- Williams C, Ellison DH, Flavell R, Booth CJ, Lu Y, Geller DS, Lifton RP: Wnk4 anism underlying adaptations to increased physical activity in controls blood pressure and potassium homeostasis via regulation of mass CKD-associated muscle wasting is not well understood. – and activity of the distal convoluted tubule. -

Mineralocorticoid-Resistant Renal Hyperkalemia Without Salt Wasting
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Kidney International, Vol. 19 (1981), pp. 716—727 Mineralocorticoid-resistant renal hyperkalemia without sal wasting (type II pseudohypoaldosteronism): Role of increased renal chloride reabsorption MORRIS SCHAMBELAN, ANTHONY SEBASTIAN, and FLOYD C. RECTOR, JR. Medical Service and Clinical Study Center, San Francisco General Hospital Medical Center, and the Department of Medicine, Cardiovascular Research Institute, and the General Clinical Research Center, University of California, San Francisco, California Mineralocorticoid-resistant renal hyperkalemia without salt Hyperkaliemie rénale resistant aux minéralocorticoides sans wasting (type II pseudohypoaldosteronism): Role of increased perte de sel (pseudohypoaldostéronisme de type II): Role de l'aug- renal chloride reabsorption. A rare syndrome has been described mentation iie Ia reabsorption de chlore. Un syndrome rare a été in which mineralocorticoid-resistant hyperkalemia of renal origin décrit dans lequel une hyperkaliemie d'origine rénale resistant occurs in the absence of glomerular insufficiency and renal aux minéralocorticoides survient en l'absence de diminution du sodium wasting and in which hyperchioremic acidosis, hyperten- debit de filtration glomerulaire et de perte rénale de sodium et sion, and hyporeninemia coexist. The primary abnormality has dans lequel une acidose hyperchioremique, une hypertension et been postulated to be a defect of the potassium secretory une hyporéninémie coexistent. L'anomalie primaire qui a été mechanism of the distal nephron. The present studies were postulée est un deficit du mécanisme de secretion de potassium carried out to investigate the mechanism of impaired renal du nephron distal. Ce travail a etC entrepris pour étudier le potassium secretion in a patient with this syndrome. -

Urinary System Diseases and Disorders
URINARY SYSTEM DISEASES AND DISORDERS BERRYHILL & CASHION HS1 2017-2018 - CYSTITIS INFLAMMATION OF THE BLADDER CAUSE=PATHOGENS ENTERING THE URINARY MEATUS CYSTITIS • MORE COMMON IN FEMALES DUE TO SHORT URETHRA • SYMPTOMS=FREQUENT URINATION, HEMATURIA, LOWER BACK PAIN, BLADDER SPASM, FEVER • TREATMENT=ANTIBIOTICS, INCREASE FLUID INTAKE GLOMERULONEPHRITIS • AKA NEPHRITIS • INFLAMMATION OF THE GLOMERULUS • CAN BE ACUTE OR CHRONIC ACUTE GLOMERULONEPHRITIS • USUALLY FOLLOWS A STREPTOCOCCAL INFECTION LIKE STREP THROAT, SCARLET FEVER, RHEUMATIC FEVER • SYMPTOMS=CHILLS, FEVER, FATIGUE, EDEMA, OLIGURIA, HEMATURIA, ALBUMINURIA ACUTE GLOMERULONEPHRITIS • TREATMENT=REST, SALT RESTRICTION, MAINTAIN FLUID & ELECTROLYTE BALANCE, ANTIPYRETICS, DIURETICS, ANTIBIOTICS • WITH TREATMENT, KIDNEY FUNCTION IS USUALLY RESTORED, & PROGNOSIS IS GOOD CHRONIC GLOMERULONEPHRITIS • REPEATED CASES OF ACUTE NEPHRITIS CAN CAUSE CHRONIC NEPHRITIS • PROGRESSIVE, CAUSES SCARRING & SCLEROSING OF GLOMERULI • EARLY SYMPTOMS=HEMATURIA, ALBUMINURIA, HTN • WITH DISEASE PROGRESSION MORE GLOMERULI ARE DESTROYED CHRONIC GLOMERULONEPHRITIS • LATER SYMPTOMS=EDEMA, FATIGUE, ANEMIA, HTN, ANOREXIA, WEIGHT LOSS, CHF, PYURIA, RENAL FAILURE, DEATH • TREATMENT=LOW NA DIET, ANTIHYPERTENSIVE MEDS, MAINTAIN FLUIDS & ELECTROLYTES, HEMODIALYSIS, KIDNEY TRANSPLANT WHEN BOTH KIDNEYS ARE SEVERELY DAMAGED PYELONEPHRITIS • INFLAMMATION OF THE KIDNEY & RENAL PELVIS • CAUSE=PYOGENIC (PUS-FORMING) BACTERIA • SYMPTOMS=CHILLS, FEVER, BACK PAIN, FATIGUE, DYSURIA, HEMATURIA, PYURIA • TREATMENT=ANTIBIOTICS, -

Role of Urinalysis in the Diagnosis of Chronic Kidney Disease (CKD)
Research and Reviews Role of Urinalysis in the Diagnosis of Chronic Kidney Disease (CKD) JMAJ 54(1): 27–30, 2011 Kunitoshi ISEKI*1 Abstract As of the end of Year 2008, 1 out of 450 people was a dialysis patient in Japan, and patients with chronic kidney disease (CKD) at stages 3 and 4 accounted for nearly 10% of the total population. An epidemiological study in Okinawa that used the introduction of dialysis treatment as the outcome revealed that the 10-year cumulative incident rate of end-stage renal disease (ESRD) was about 3% of the participants who were positive (Ն 1ϩ) for both proteinuria and hematuria, while there was hardly any difference between those who were positive for hematuria alone and those who were negative for both proteinuria and hematuria. When the incidence of ESRD (dialysis introduction) was examined in relation to the severity of proteinuria (5 grades ranging from [Ϫ] to [Ն 3ϩ]) as determined by dipstick, the cumulative incidence rate during the 17-year observation period was 16% for proteinuria (Ն 3ϩ) and about 7% for proteinuria (2ϩ). In contrast, among participants who were negative for proteinuria, the rate of dialysis introduction in 10 years is about 1 out of 1 million. The CKD Practice Guide of the Japanese Society of Nephrology recommends referral to a nephrologist when a case meets any of the following 3 criteria: 1) 0.5g/g creatinine or higher, or proteinuria (Ն 2ϩ), 2) an estimated glomerular filtration rate of less than 50ml/min/1.73m2, or 3) positive results (Ն 1ϩ) for both proteinuria and hematuria tests. -

Renal Papillary Necrosis in Infancy PETER HUSBAND* and K
Arch Dis Child: first published as 10.1136/adc.48.2.116 on 1 February 1973. Downloaded from Archives of Disease in Childhood, 1973, 48, 116. Renal papillary necrosis in infancy PETER HUSBAND* and K. A. HOWLETT From the Departments of Paediatrics and Radiology, Charing Cross Group of Hospitals, Fulham Hospital, London Husband, P., and Howlett, K. A. (1973). Archives of Disease in Childhood, 48, 116. Renal papillary necrosis in infancy. Two infants developed renal papillary necrosis after acute illnesses associated with dehydration. After a short oliguric phase there was a longer phase characterized by impaired urinary concen- tration, hyponatraemia, metabolic acidosis, and a raised blood urea. Intravenous urograms showed contrast collecting in the papillae, with subsequent sinus formation extending into the medulla. In one case impaired urinary concentration was still present 21 months after the initial illness. Renal papillary necrosis was originally described admitted to hospital 9 days later after he had been by Friedreich (1877) in a man aged 70 years. found in his cot, grey, with respiratory distress and a Since then the majority of further cases reported high temperature. 3 days before he had diarrhoea and have also been in adults. It has been thought to vomiting for 24 hours but subsequently tolerated feeds be uncommon and usually to have a fatal outcome of full cream Cow and Gate milk, 180 ml 5 times a day. He was again extremely ill: temperature 40 3 °C, pale, copyright. in infancy and childhood (Davies, Kennedy, and cyanosed with a rapid respiratory rate, but not dehydra- Roberts, 1969). In the newborn infant renal ted. -

Risk of Renal Function Decline in Patients with Ketamine-Associated Uropathy
International Journal of Environmental Research and Public Health Article Risk of Renal Function Decline in Patients with Ketamine-Associated Uropathy Shih-Hsiang Ou 1,2,3, Ling-Ying Wu 4, Hsin-Yu Chen 1,2, Chien-Wei Huang 1,2, Chih-Yang Hsu 1,2, Chien-Liang Chen 1,2 , Kang-Ju Chou 1,2, Hua-Chang Fang 1,2 and Po-Tsang Lee 1,2,* 1 Division of Nephrology, Department of Internal Medicine, Kaohsiung Veterans General Hospital, Kaohsiung 813, Taiwan; [email protected] (S.-H.O.); [email protected] (H.-Y.C.); [email protected] (C.-W.H.); [email protected] (C.-Y.H.); [email protected] (C.-L.C.); [email protected] (K.-J.C.); [email protected] (H.-C.F.) 2 Faculty of Medicine, School of Medicine, National Yang-Ming University, Taipei 112, Taiwan 3 Graduate Institute of Clinical Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung 807, Taiwan 4 Department of Obstetrics and Gynecology, Kaohsiung Chang Gung Memorial Hospital, Kaohsiung 833, Taiwan; [email protected] * Correspondence: [email protected]; Tel.: +886-7342-2121 (ext. 8090) Received: 8 August 2020; Accepted: 29 September 2020; Published: 4 October 2020 Abstract: Ketamine-associated diseases have been increasing with the rise in ketamine abuse. Ketamine-associated uropathy is one of the most common complications. We investigated the effects of ketamine-associated uropathy on renal health and determined predictors of renal function decline in chronic ketamine abusers. This retrospective cohort study analyzed 51 patients (22 with ketamine- associated hydronephrosis and 29 with ketamine cystitis) from Kaohsiung Veterans General Hospital in Taiwan. -

Hematuria in the Child
Hematuria and Proteinuria in the Pediatric Patient Laurie Fouser, MD Pediatric Nephrology Swedish Pediatric Specialty Care Hematuria in the Child • Definition • ³ 1+ on dipstick on three urines over three weeks • 5 RBCs/hpf on three fresh urines over three weeks • Prevalence • 4-6% for microscopic hematuria on a single specimen in school age children • 0.3-0.5% on repeated specimens Sources of Hematuria • Glomerular or “Upper Tract” – Dysmorphic RBCs and RBC casts – Tea or cola colored urine – Proteinuria, WBC casts, renal tubular cells • Non-Glomerular or “Lower Tract” – RBCs have normal morphology – Clots/ Bright red or pink urine The Glomerular Capillary Wall The Glomerular Capillary Wall Glomerular Causes of Hematuria • Benign or self-limiting – Benign Familial Hematuria – Exercise-Induced Hematuria – Fever-Induced Hematuria Glomerular Causes of Hematuria • Acute Glomerular Disease – Poststreptococcal/ Postinfectious – Henoch-Schönlein Purpura – Sickle Cell Disease – Hemolytic Uremic Syndrome Glomerular Causes of Hematuria • Chronic Glomerular Disease – IgA Nephropathy – Henoch-Schönlein Purpura or other Vasculitis – Alport Syndrome – SLE or other Collagen Vascular Disease – Proliferative Glomerulonephritis Non-Glomerular Hematuria • Extra-Renal • UTI • Benign urethralgia +/- meatal stenosis • Calculus • Vesicoureteral Reflux, Hydronephrosis • Foreign body • Rhabdomyosarcoma • AV M • Coagulation disorder Non-Glomerular Hematuria • Intra-Renal • Hypercalciuria • Polycystic Kidney Disease • Reflux Nephropathy with Renal Dysplasia • -
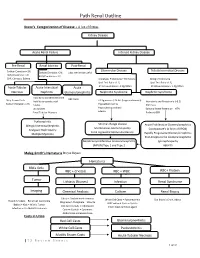
Path Renal Outline
Path Renal Outline Krane’s Categorization of Disease + A lot of Extras Kidney Disease Acute Renal Failure Intrinsic Kidney Disease Pre‐Renal Renal Intrinsic Post‐Renal Sodium Excretion <1% Glomerular Disease Tubulointerstitial Disease Sodium Excretion < 1% Sodium Excretion >2% Labs aren’t that useful BUN/Creatinine > 20 BUN/Creatinine < 10 CHF, Cirrhosis, Edema Urinalysis: Proteinuria + Hematuria Benign Proteinuria Spot Test Ratio >1.5, Spot Test Ratio <1.5, Acute Tubular Acute Interstitial Acute 24 Urine contains > 2.0g/24hrs 24 Urine contains < 1.0g/24hrs Necrosis Nephritis Glomerulonephritis Nephrotic Syndrome Nephritic Syndrome Inability to concentrate Urine RBC Casts Dirty Brown Casts Inability to secrete acid >3.5g protein / 24 hrs (huge proteinuria) Hematuria and Proteinuria (<3.5) Sodium Excretion >2% Edema Hypoalbuminemia RBC Casts Hypercholesterolemia Leukocytes Salt and Water Retention = HTN Focal Tubular Necrosis Edema Reduced GFR Pyelonephritis Minimal change disease Allergic Interstitial Nephritis Acute Proliferative Glomerulonephritis Membranous Glomerulopathy Analgesic Nephropathy Goodpasture’s (a form of RPGN) Focal segmental Glomerulosclerosis Rapidly Progressive Glomerulonephritis Multiple Myeloma Post‐Streptococcal Glomerulonephritis Membranoproliferative Glomerulonephritis IgA nephropathy (MPGN) Type 1 and Type 2 Alport’s Meleg‐Smith’s Hematuria Break Down Hematuria RBCs Only RBC + Crystals RBC + WBC RBC+ Protein Tumor Lithiasis (Stones) Infection Renal Syndrome Imaging Chemical Analysis Culture Renal Biopsy Calcium -

Acute Tubular Necrosıs After Nephrectomy: Case Presentatıon
Case Report JOJ Case Stud Volume 7 Issue 1 - May 2018 Copyright © All rights are reserved by Ebru Canakci DOI: 10.19080/JOJCS.2018.07.555703 Acute Tubular Necrosıs after Nephrectomy: Case Presentatıon Ebru Canakci1*, Ahmet Karatas2, Ahmet Gultekin1, Zubeyir Cebeci1, Ilker Coskun1 and Anıl Kılınc1 1Department of Anaesthesiology and Reanimation, Ordu University, Turkey 2Department of Internal Medicine & Nephrology, Ordu University, Turkey Submission: May 07, 2018; Published: May 18, 2018 *Corresponding author: Ebru Canakci, Faculty of Medicine, Department of Anaesthesiology and Reanimation, Ordu University, Ordu, Turkey, Email: Abstract ATN, contrary to prerenal azotemia, is not immediately cured upon the recovery of renal perfusion. In its severe form, renal hypoperfusion leads Acute renal failure (ARF) has a clinical presentation with declining renal function and glomerular filtration rate within hours-days. Ischemic trauma, severe hypovolemia, sepsis and severe burns. Acute kidney injury (AKI) is one of the frequently encountered causes of morbidity and mortalityto bilateral in renal hospitals. cortical The necrosis aim of this and study irreversible is to present renal the insufficiency. case with ATN Ischemic after major ATN often surgery develops and subsequent as a result permanent of major surgical kidney injuryintervention, in light of the information from the literature. Keywords: Nephrectomy; Acute Tubular Necrosis; Hemodialysis Introductıon and Objectıve to endogenous or exogenous toxins. Toxins cause intrarenal Acute renal failure (ARF) has a clinical presentation with vasoconstriction, direct tubular toxicity and/or intratubular obstruction and thus lead to ARF [4]. Acute kidney injury (AKI) hours-days. Although there are several differences in the declining renal function and glomerular filtration rate within is one of the frequently encountered causes of morbidity and mortality in hospitals.