Type of Drug &
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

United States July 2016 2 Table of Contents
Deuterium Labelled Compounds United States July 2016 2 Table of Contents International Distributors 3 Corporate Overview 4 General Information 5 Pricing and Payment 5 Quotations 5 Custom Synthesis 5 Shipping 5 Quality Control 6 Quotations 6 Custom Synthesis 6 Shipping 6 Quality Control 6 Chemical Abstract Service Numbers 6 Handling Hazardous Compounds 6 Our Products are Not Intended for Use in Humans 7 Limited Warranty 7 Packaging Information 7 Alphabetical Listings 8 Stock Clearance 236 Products by Category 242 n-Alkanes 243 α-Amino Acids, N-Acyl α-Amino Acids, N-t-BOC Protected α-Amino Acid 243 and N-FMOC Protected α-Amino Acids Buffers and Reagents for NMR Studies 245 Detergents 245 Environmental Standards 246 Fatty Acids and Fatty Acid Esters 249 Flavours and Fragrances 250 Gases 253 Medical Research Products 254 Nucleic Acid Bases and Nucleosides 255 Pesticides and Pesticide Metabolites 256 Pharmaceutical Standards 257 Polyaromatic Hydrocarbons (PAHs), Alkyl-PAHs, Amino-PAHs, 260 Hydroxy-PAHs and Nitro-PAHs Polychlorinated Biphenyls (PCBs) 260 Spin Labels 261 Steroids 261 3 International Distributors C Beijng Zhenxiang H EQ Laboratories GmbH Australia K Technology Company Graf-von-Seyssel-Str. 10 Rm. 15A01, Changyin Bld. 86199 Augsburg Austria H No. 88, YongDingLu Rd. Germany Beijing 100039 Tel.: (49) 821 71058246 Belgium J China Fax: (49) 821 71058247 Tel.: (86) 10-58896805 [email protected] China C Fax: (86) 10-58896158 www.eqlabs.de Czech Republic H [email protected] Germany, Austria, China Czech Republic, Greece, Denmark I Hungary, -

Campro Catalog Stable Isotope
Introduction & Welcome Dear Valued Customer, We are pleased to present to you our Stable Isotopes Catalog which contains more than three thousand (3000) high quality labeled compounds. You will find new additions that are beneficial for your research. Campro Scientific is proud to work together with Isotec, Inc. for the distribution and marketing of their stable isotopes. We have been working with Isotec for more than twenty years and know that their products meet the highest standard. Campro Scientific was founded in 1981 and we provide services to some of the most prestigious universities, research institutes and laboratories throughout Europe. We are a research-oriented company specialized in supporting the requirements of the scientific community. We are the exclusive distributor of some of the world’s leading producers of research chemicals, radioisotopes, stable isotopes and environmental standards. We understand the requirements of our customers, and work every day to fulfill them. In working with us you are guaranteed to receive: - Excellent customer service - High quality products - Dependable service - Efficient distribution The highly educated staff at Campro’s headquarters and sales office is ready to assist you with your questions and product requirements. Feel free to call us at any time. Sincerely, Dr. Ahmad Rajabi General Manager 180/280 = unlabeled 185/285 = 15N labeled 181/281 = double labeled (13C+15N, 13C+D, 15N+18O etc.) 186/286 = 12C labeled 182/282 = d labeled 187/287 = 17O labeled 183/283 = 13C labeleld 188/288 = 18O labeled 184/284 = 16O labeled, 14N labeled 189/289 = Noble Gases Table of Contents Ordering Information.................................................................................................. page 4 - 5 Packaging Information .............................................................................................. -

Safety and Efficacy of Ibutilide in Cardioversion of Atrial Flutter And
J Am Board Fam Med: first published as 10.3122/jabfm.2011.01.080096 on 5 January 2011. Downloaded from CLINICAL REVIEW Safety and Efficacy of Ibutilide in Cardioversion of Atrial Flutter and Fibrillation Madhuri Nair, MD, Lekha K. George, MD, and Santhosh K. G. Koshy, MD This article reviews the safety and efficacy of ibutilide for use in patients with atrial fibrillation and flut- ter. Ibutilide, a class III antiarrhythmic agent, is primarily used for conversion of atrial flutter and fi- brillation and is a good alternative to electrical cardioversion. Ibutilide has a conversion rate of up to 75% to 80% in recent-onset atrial fibrillation and flutter; the conversion rate is higher for atrial flutter than for atrial fibrillation. It is also safe in the conversion of chronic atrial fibrillation/flutter among patients receiving oral amiodarone therapy. Ibutilide pretreatment facilitates transthoracic defibrilla- tion and decreases the energy requirement of electrical cardioversion by both monophasic and biphasic shocks. Pretreatment with ibutilide before electrical defibrillation has a conversion rate of 100% com- pared with 72% with no pretreatment. Ibutilide is also safe and efficient in the treatment of atrial fibril- lation in patients who have had cardiac surgery, and in accessory pathway–mediated atrial fibrillation where the conversion rate of ibutilide is as high as 95%. There is up to a 4% risk of torsade de pointes and a 4.9% risk of monomorphic ventricular tachycardia. Hence, close monitoring in an intensive care unit setting is warranted during and at least for 4 hours after drug infusion. The anticoagulation strat- egy is the same as for any other mode of cardioversion.(J Am Board Fam Med 2011;24:86–92.) Keywords: Antiarrhythmics, Arrhythmia, Atrial Fibrillation, Cardiovascular Disorders, Cardioversion, Drug Ther- copyright. -

Hypothalamic Principles Necessary for the Release of Ovulating Hormone from the Adenohypophysis
EFFECT OF AMINOGLUTETHIMIDE ON REPRODUCTIVE PROCESSES IN FEMALE RATS W. J. EVERSOLE and D. J. THOMPSON Department of Life Sciences, Indiana State University, Terre Haute, Indiana 47809, U.S.A. (Received "òlst January 1974) Summary. Adult female rats injected subcutaneously with 100 mg aminoglutethimide phosphate (AGP)/kg/day for 4 weeks failed to become pregnant when placed in cohabitation with males during the last 2 weeks of the injection period. Lower doses depressed the fertility rate and reduced the litter size: the average litter size of three of eleven rats given 50 mg/kg/day was 4\m=.\7 young and of four of eleven rats given 25 mg was 7\m=.\0 young. Twenty-nine controls averaged 10\m=.\1/litter. Doses of 100 mg AGP/kg/day stopped vaginal cycling, prevented ovulation in adults, and delayed dissolution of the vaginal membrane in pubertal rats. Histological studies of the ovaries from rats with initial ages of 17 or 21 days which were injected with 25 to 100 mg AGP/kg/day for 2 weeks showed an increase in the number and size of vesicular follicles. Treatment of 27-day-old rats with 25 mg/kg/day for 2 weeks reduced the number of CL and three of four rats given 50 mg had ovaries lacking such bodies; all control ovaries in this group contained CL. These findings are taken as evidence that AGP inhibits ovulation but does not prevent follicular maturation. INTRODUCTION Many pharmacological compounds originally developed as anaesthetics, tranquillizers and anticonvulsants modify endocrine structures and functions (see Gaunt, Chart & Renzi, 1965). -

Supplementary Materials
Supplementary Materials Table S1. The significant drug pairs in potential DDIs examined by the two databases. Micromedex Drugs.com List of drugs paired PK-PD Mechanism details 1. Amiodarone— PD Additive QT-interval prolongation Dronedarone 2. Amiodarone— PK CYP3A inhibition by Ketoconazole Ketoconazole 3. Ciprofloxacin— PD Additive QT-interval prolongation Dronedarone 4. Cyclosporine— PK CYP3A inhibition by Cyclosporine Dronedarone 5. Dronedarone— PK CYP3A inhibition by Erythromycin Erythromycin 6. Dronedarone— PD Additive QT-interval prolongation Flecainide 7. Dronedarone— PK CYP3A4 inhibition by Itraconazole Itraconazole 8. Dronedarone— PK Contraindication Major CYP3A inhibition by Ketoconazole Ketoconazole 9. Dronedarone— PD Additive QT-interval prolongation Procainamide PD 10. Dronedarone—Sotalol Additive QT-interval prolongation 11. Felodipine— PK CYP3A inhibition by Itraconazole Itraconazole 12. Felodipine— PK CYP3A inhibition by Ketoconazole Ketoconazole 13. Itraconazole— PK CYP3A inhibition by Itraconazole Nisoldipine 14. Ketoconazole— PK CYP3A inhibition by Ketoconazole Nisoldipine 15. Praziquantel— PK CYP induction by Rifampin Rifampin PD 1. Amikacin—Furosemide Additive or synergistic toxicity 2. Aminophylline— Decreased clearance of PK Ciprofloxacin Theophylline by Ciprofloxacin 3. Aminophylline— PK Decreased hepatic metabolism Mexiletine 4. Amiodarone— PD Additive effects on QT interval Ciprofloxacin 5. Amiodarone—Digoxin PK P-glycoprotein inhibition by Amiodarone 6. Amiodarone— PD, PK Major Major Additive effects on QT Erythromycin prolongation, CYP3A inhibition by Erythromycin 7. Amiodarone— PD, PK Flecainide Antiarrhythmic inhibition by Amiodarone, CYP2D inhibition by Amiodarone 8. Amiodarone— PK CYP3A inhibition by Itraconazole Itraconazole 9. Amiodarone— PD Antiarrhythmic inhibition by Procainamide Amiodarone 10. Amiodarone— PK CYP induction by Rifampin Rifampin PD Additive effects on refractory 11. Amiodarone—Sotalol potential 12. Amiodarone— PK CYP3A inhibition by Verapamil Verapamil 13. -

Mechanisms of Action of Antiepileptic Drugs
Review Mechanisms of action of antiepileptic drugs Epilepsy affects up to 1% of the general population and causes substantial disability. The management of seizures in patients with epilepsy relies heavily on antiepileptic drugs (AEDs). Phenobarbital, phenytoin, carbamazepine and valproic acid have been the primary medications used to treat epilepsy for several decades. Since 1993 several AEDs have been approved by the US FDA for use in epilepsy. The choice of the AED is based primarily on the seizure type, spectrum of clinical activity, side effect profile and patient characteristics such as age, comorbidities and concurrent medical treatments. Those AEDs with broad- spectrum activity are often found to exert an action at more than one molecular target. This article will review the proposed mechanisms of action of marketed AEDs in the US and discuss the future of AEDs in development. 1 KEYWORDS: AEDs anticonvulsant drugs antiepileptic drugs epilepsy Aaron M Cook mechanism of action seizures & Meriem K Bensalem-Owen† The therapeutic armamentarium for the treat- patients with refractory seizures. The aim of this 1UK HealthCare, 800 Rose St. H-109, ment of seizures has broadened significantly article is to discuss the past, present and future of Lexington, KY 40536-0293, USA †Author for correspondence: over the past decade [1]. Many of the newer AED pharmacology and mechanisms of action. College of Medicine, Department of anti epileptic drugs (AEDs) have clinical advan- Neurology, University of Kentucky, 800 Rose Street, Room L-455, tages over older, so-called ‘first-generation’ First-generation AEDs Lexington, KY 40536, USA AEDs in that they are more predictable in their Broadly, the mechanisms of action of AEDs can Tel.: +1 859 323 0229 Fax: +1 859 323 5943 dose–response profile and typically are associ- be categorized by their effects on the neuronal [email protected] ated with less drug–drug interactions. -

Stems for Nonproprietary Drug Names
USAN STEM LIST STEM DEFINITION EXAMPLES -abine (see -arabine, -citabine) -ac anti-inflammatory agents (acetic acid derivatives) bromfenac dexpemedolac -acetam (see -racetam) -adol or analgesics (mixed opiate receptor agonists/ tazadolene -adol- antagonists) spiradolene levonantradol -adox antibacterials (quinoline dioxide derivatives) carbadox -afenone antiarrhythmics (propafenone derivatives) alprafenone diprafenonex -afil PDE5 inhibitors tadalafil -aj- antiarrhythmics (ajmaline derivatives) lorajmine -aldrate antacid aluminum salts magaldrate -algron alpha1 - and alpha2 - adrenoreceptor agonists dabuzalgron -alol combined alpha and beta blockers labetalol medroxalol -amidis antimyloidotics tafamidis -amivir (see -vir) -ampa ionotropic non-NMDA glutamate receptors (AMPA and/or KA receptors) subgroup: -ampanel antagonists becampanel -ampator modulators forampator -anib angiogenesis inhibitors pegaptanib cediranib 1 subgroup: -siranib siRNA bevasiranib -andr- androgens nandrolone -anserin serotonin 5-HT2 receptor antagonists altanserin tropanserin adatanserin -antel anthelmintics (undefined group) carbantel subgroup: -quantel 2-deoxoparaherquamide A derivatives derquantel -antrone antineoplastics; anthraquinone derivatives pixantrone -apsel P-selectin antagonists torapsel -arabine antineoplastics (arabinofuranosyl derivatives) fazarabine fludarabine aril-, -aril, -aril- antiviral (arildone derivatives) pleconaril arildone fosarilate -arit antirheumatics (lobenzarit type) lobenzarit clobuzarit -arol anticoagulants (dicumarol type) dicumarol -

Membrane Stabilizer Medications in the Treatment of Chronic Neuropathic Pain: a Comprehensive Review
Current Pain and Headache Reports (2019) 23: 37 https://doi.org/10.1007/s11916-019-0774-0 OTHER PAIN (A KAYE AND N VADIVELU, SECTION EDITORS) Membrane Stabilizer Medications in the Treatment of Chronic Neuropathic Pain: a Comprehensive Review Omar Viswanath1,2,3 & Ivan Urits4 & Mark R. Jones4 & Jacqueline M. Peck5 & Justin Kochanski6 & Morgan Hasegawa6 & Best Anyama7 & Alan D. Kaye7 Published online: 1 May 2019 # Springer Science+Business Media, LLC, part of Springer Nature 2019 Abstract Purpose of Review Neuropathic pain is often debilitating, severely limiting the daily lives of patients who are affected. Typically, neuropathic pain is difficult to manage and, as a result, leads to progression into a chronic condition that is, in many instances, refractory to medical management. Recent Findings Gabapentinoids, belonging to the calcium channel blocking class of drugs, have shown good efficacy in the management of chronic pain and are thus commonly utilized as first-line therapy. Various sodium channel blocking drugs, belonging to the categories of anticonvulsants and local anesthetics, have demonstrated varying degrees of efficacy in the in the treatment of neurogenic pain. Summary Though there is limited medical literature as to efficacy of any one drug, individualized multimodal therapy can provide significant analgesia to patients with chronic neuropathic pain. Keywords Neuropathic pain . Chronic pain . Ion Channel blockers . Anticonvulsants . Membrane stabilizers Introduction Neuropathic pain, which is a result of nervous system injury or lives of patients who are affected. Frequently, it is difficult to dysfunction, is often debilitating, severely limiting the daily manage and as a result leads to the progression of a chronic condition that is, in many instances, refractory to medical This article is part of the Topical Collection on Other Pain management. -

NACE Bromine Chemistry Review Paper
25 YEARS OF BROMINE CHEMISTRY IN INDUSTRIAL WATER SYSTEMS: A REVIEW Christopher J. Nalepa Albemarle Corporation P.O. Box 14799 Baton Rouge, LA 70898 ABSTRACT Bromine chemistry is used to great advantage in nature for fouling control by a number of sessile marine organisms such as sponges, seaweeds, and bryozoans. Such organisms produce small quantities of brominated organic compounds that effectively help keep their surfaces clean of problem bacteria, fungi, and algae. For over two decades, bromine chemistry has been used to similar advantage in the treatment of industrial water systems. The past several years in particular has seen the development of several diverse bromine product forms – one-drum stabilized bromine liquids, all-bromine hydantoin solids, and pumpable gels. The purpose of this paper is to review the development of bromine chemistry in industrial water treatment, discuss characteristics of the new product forms, and speculate on future developments. Keywords: Oxidizing biocide, bleach, bromine, bromine chemistry, sodium hypobromite, activated sodium bromide, Bromochlorodimethylhydantoin, Bromochloromethyethylhydantoin, Dibromodi- methylhydantoin,, BCDMH, BCMEH, DBDMH, stabilized bromine chloride, stabilized hypobromite INTRODUCTION Sessile marine organisms generate metabolites to ward off predators and deter attachment of potential micro- and macrofoulants. Sponges, algae, and bryozoans for example, produce a rich variety of bromine-containing compounds that exhibit antifoulant properties (Fig. 1).1,2,3 Scientists are actively studying these organisms to understand how they maintain surfaces that are relatively clean and slime- free.4 Brominated furanones isolated from the red algae Delisea pulchra, for example, have been found to interfere with the chemical signals (acylated homoserine lactones) that bacteria use to communicate with one another to produce biofilms.5,6 This work may eventually lead to more effective control of microorganisms in a number of industries such as industrial water treatment, oil and gas production, health care, etc. -
Keeping up with the Pace of Antiarrhythmic Drugs ANNMARIE PALATNIK, APN,BC, MSN Coordinator of Continuing Education • Virtua Health • Marlton, N.J
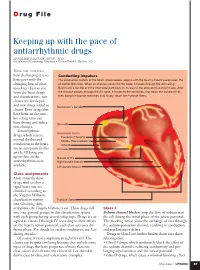
D rug File Keeping up with the pace of antiarrhythmic drugs ANNMARIE PALATNIK, APN,BC, MSN Coordinator of Continuing Education • Virtua Health • Marlton, N.J. HAVE YOU NOTICED how challenging it is to Conducting impulses keep pace with the The conduction system of the heart, shown below, begins with the heart’s natural pacemaker, the changing beat of phar- sinoatrial (SA) node. When an impulse leaves the SA node, it travels through the atria along macology? Just as you Bachmann’s bundle and the internodal pathways on its way to the atrioventricular (AV) node. After learn the latest drugs the impulse passes through the AV node, it travels to the ventricles, first down the bundle of His, and classifications, new then along the bundle branches and, finally, down the Purkinje fibers. classes are developed and new drugs added to Bachmann’s bundle classes. Even drugs that have been on the mar- ket a long time can have dosing and indica- SA node tion changes. Antiarrhythmic Internodal tracts drugs, which restore Posterior (Thorel’s) normal rhythm and Middle (Wenckebach’s) conduction to the heart, Anterior are no exception. In this AV node article, I’ll bring you up-to-date on the Bundle of His antiarrhythmics now Right bundle branch available. Left bundle branch Class assignments Most antiarrhythmic drugs used to slow a rapid heart rate are classified according to the Vaughn Williams classification system Purkinje fibers (see Classifying Anti- arrhythmics the Vaughn Williams way). These drugs fall Class I into four general groups in this classification system Sodium channel blockers stop the flow of sodium into with each group having several subgroups. -

Vitamin D Receptor Promotes Healthy Microbial Metabolites
www.nature.com/scientificreports OPEN Vitamin D receptor promotes healthy microbial metabolites and microbiome Ishita Chatterjee1, Rong Lu1, Yongguo Zhang1, Jilei Zhang1, Yang Dai 2, Yinglin Xia1 ✉ & Jun Sun 1 ✉ Microbiota derived metabolites act as chemical messengers that elicit a profound impact on host physiology. Vitamin D receptor (VDR) is a key genetic factor for shaping the host microbiome. However, it remains unclear how microbial metabolites are altered in the absence of VDR. We investigated metabolites from mice with tissue-specifc deletion of VDR in intestinal epithelial cells or myeloid cells. Conditional VDR deletion severely changed metabolites specifcally produced from carbohydrate, protein, lipid, and bile acid metabolism. Eighty-four out of 765 biochemicals were signifcantly altered due to the Vdr status, and 530 signifcant changes were due to the high-fat diet intervention. The impact of diet was more prominent due to loss of VDR as indicated by the diferences in metabolites generated from energy expenditure, tri-carboxylic acid cycle, tocopherol, polyamine metabolism, and bile acids. The efect of HFD was more pronounced in female mice after VDR deletion. Interestingly, the expression levels of farnesoid X receptor in liver and intestine were signifcantly increased after intestinal epithelial VDR deletion and were further increased by the high-fat diet. Our study highlights the gender diferences, tissue specifcity, and potential gut-liver-microbiome axis mediated by VDR that might trigger downstream metabolic disorders. Metabolites are the language between microbiome and host1. To understand how host factors modulate the microbiome and consequently alter molecular and physiological processes, we need to understand the metabo- lome — the collection of interacting metabolites from the microbiome and host. -

Pharmaceuticals As Environmental Contaminants
PharmaceuticalsPharmaceuticals asas EnvironmentalEnvironmental Contaminants:Contaminants: anan OverviewOverview ofof thethe ScienceScience Christian G. Daughton, Ph.D. Chief, Environmental Chemistry Branch Environmental Sciences Division National Exposure Research Laboratory Office of Research and Development Environmental Protection Agency Las Vegas, Nevada 89119 [email protected] Office of Research and Development National Exposure Research Laboratory, Environmental Sciences Division, Las Vegas, Nevada Why and how do drugs contaminate the environment? What might it all mean? How do we prevent it? Office of Research and Development National Exposure Research Laboratory, Environmental Sciences Division, Las Vegas, Nevada This talk presents only a cursory overview of some of the many science issues surrounding the topic of pharmaceuticals as environmental contaminants Office of Research and Development National Exposure Research Laboratory, Environmental Sciences Division, Las Vegas, Nevada A Clarification We sometimes loosely (but incorrectly) refer to drugs, medicines, medications, or pharmaceuticals as being the substances that contaminant the environment. The actual environmental contaminants, however, are the active pharmaceutical ingredients – APIs. These terms are all often used interchangeably Office of Research and Development National Exposure Research Laboratory, Environmental Sciences Division, Las Vegas, Nevada Office of Research and Development Available: http://www.epa.gov/nerlesd1/chemistry/pharma/image/drawing.pdfNational