Salt-Affected Areas
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Soil Salinity in Agricultural Systems: the Basics
Soil Salinity in Agricultural Systems: The Basics Jeffrey L. Ullman Agricultural & Biological Engineering University of Florida Strategies for Minimizing Salinity Problems and Optimizing Crop Production In-Service Training, Hastings, FL March 26, 2013 What is salt? What is Salt? . Salts are more than just sodium chloride (NaCl) . Salts consist of anions and cations . In terms of soil and irrigation water these generally include: Cations Anions Sodium Na+ Chlorides Cl- 2+ 2- Magnesium Mg Sulfates SO4 2+ 2- Calcium Ca Carbonates CO3 - Bicarbonates HCO3 What is Salt? . Other salts in agriculture + Potassium (K ) - Nitrate (NO3 ) Boron (B) • Often as boric acid (H3BO3, often written as B(OH)3) • Can form salts such as sodium borate (borax; Na2B4O7) Photo: Georgia Agriculture What is Salt? H O(l) NaCl(s) 2 Na+(aq) + Cl-(aq) (aq) indicates that Na+ and Cl- are hydrated ions Sodium sulfate Magnesium carbonate Source: Averill and Eldredge (2007) Types of Salts Some common salts NaCl Sodium chloride Table salt (halite) CO 2- 3 KCl Potassium chloride Muriate of potash Na+ 2- NaHCO3 Sodium bicarbonate Baking soda (nahcolite) SO4 - Cl CaSO4 Calcium sulfate Gypsum + K CaCO3 Calcium carbonate Calcite 2+ Ca MgSO Magnesium sulfate Epsom salt (epsomite) Mg2+ 4 K2SO4 Potassium sulfate Sulfate of potash (arcanite) HCO - 3 Glauber’s salt (thenardite Na SO Sodium sulfate 2 4 and mirabilite) Gypsum Calcite Thenardite Sources of Salt . Dissolution of parent rock material . Irrigation water . Saline groundwater . Fertilizers . Manure . Seawater intrusion Photo: J. Ullman Saline Soils . Accumulation of salts known as salination . Can occur in diverse types of soil with different physical, chemical and hydrologic properties Photo: USDA-NRCS Saline Soils . -

Soil Salinity Type Effects on the Relationship Betweenthe Electrical
sustainability Article Soil Salinity Type Effects on the Relationship between the Electrical Conductivity and Salt Content for 1:5 Soil-to-Water Extract Amin I. Ismayilov 1, Amrakh I. Mamedov 2,* , Haruyuki Fujimaki 2 , Atsushi Tsunekawa 2 and Guy J. Levy 3 1 Institute of Soil Science and Agrichemistry, Azerbaijan National Academy of Sciences (ANAS), Baku AZ1073, Azerbaijan; [email protected] 2 Arid Land Research Center, Tottori University, Tottori 680-0001, Japan; [email protected] (H.F.); [email protected] (A.T.) 3 Institute of Soil, Water and Environmental Sciences, ARO, Rishon LeZion 7505101, Israel; [email protected] * Correspondence: [email protected] Abstract: Soil salinity severely affects soil ecosystem quality and crop production in semi-arid and arid regions. A vast quantity of data on soil salinity has been collected by research organizations of the Commonwealth of Independent States (CIS, formerly USSR) and many other countries over the last 70 years, but using them in the current international network (irrigation and reclamation strategy) is complicated. This is because in the CIS countries salinity was expressed by total soluble salts as a percentage on a dry-weight basis (total soluble salts, TSS, %) and eight salinity types − 2− − + (chemistry) determined by the ratios of the anions and cations (Cl , SO4 , HCO3 , and Na , Ca2+, Mg2+) in diluted soil water extract (soil/water = 1:5) without assessing electrical conductivity (EC). Measuring the EC (1:5) is more convenient, yet EC is not only affected by the concentration Citation: Ismayilov, A.I.; Mamedov, but also characteristics of the ions and the salinity chemistry. -

Status of Soil Salinity in California
it a major item in his joint presentation percent of construction, operation, and Although progress has been made, the to Congress and meeting with former maintenance costs. Basin states see the need for expanded President Nixon in 1972. In 1975, the Forum recommended wa- salinity control to maintain the numeric ter quality standards for salinity, includ- Proposed solutions criteria. Bills now before Congress would ing numeric criteria of 723 mg/L below authorize five additional salinity control The salinity problem has the potential to Hoover Dam, 747 mg/L below Parker units to be constructed by the Depart- cause lengthy legal and political battles Dam, and 879 mg/L at Imperial Dam. ment of the Interior, give the US. De- between the Upper and Lower Basin Their proposal also called for prompt partment of Agriculture specific author- states. The Lower Basin wants to pre- construction of the salinity control units ity for a program of on-farm Colorado vent salinity increases that would result authorized by P.L. 93-320, construction River salinity control measures in coop- from further upstream development; Up- of additional units upon completion of eration with local landowners, and pro- per Basin states are concerned that the planning reports, implementation of on- vide for 25 percent of the construction salinity issue could prevent future in- farm water management practices to costs to be paid by the Basin states. creases in their water use. control salinity, limitations on industrial In other efforts to control the river’s The states began to work together and municipal discharges, use of saline salinity, the Basin states have adopted a and with the federal government in the water for industrial purposes, and the policy calling for a no-salt return from late 1960s, and in the early 1970s several inclusion of the salinity components of industrial discharges and limiting the steps were taken to deal with the prob- water quality management plans devel- incremental increase permitted from lems. -
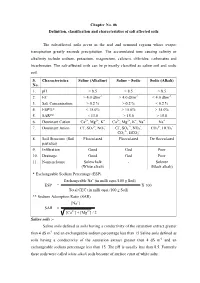
Chapter No. 06 Definition, Classification and Characteristics of Salt Affected Soils
Chapter No. 06 Definition, classification and characteristics of salt affected soils The salt-affected soils occur in the arid and semiarid regions where evapo- transpiration greatly exceeds precipitation. The accumulated ions causing salinity or alkalinity include sodium, potassium, magnesium, calcium, chlorides, carbonates and bicarbonates. The salt-affected soils can be primarily classified as saline soil and sodic soil. S. Characteristics Saline (Alkaline) Saline – Sodic Sodic (Alkali) No. 1. pH < 8.5 > 8.5 > 8.5 2. EC > 4.0 dSm-1 > 4.0 dSm-1 < 4.0 dSm-1 3. Salt Concentration > 0.2 % > 0.2 % < 0.2 % 4. ESP%* < 15.0% > 15.0% > 15.0% 5. SAR** < 13.0 > 15.0 > 15.0 6. Dominant Cation Ca2+, Mg2+, K+ Ca2+, Mg2+, K+, Na+ Na+ - 2- - - 2- - 2- - 7. Dominant Anion Cl , SO4 , NO3 Cl , SO4 , NO3 , CO3 , HCO3 2- - CO3 , HCO3 8. Soil Structure (Soil Flocculated Flocculated De flocculated particles) 9. Infiltration Good God Poor 10. Drainage Good God Poor 11. Nomenclature Solenchalk - Solentz (White alkali) (Black alkali) * Exchangeable Sodium Percentage (ESP) Exchangeable Na+ (in milli equi./100 g Soil) ESP = X 100 Total CEC (in milli equi./100 g Soil) ** Sodium Adsorption Ratio (SAR) [Na+] SAR = [Ca2+] + [Mg2+] / 2 Saline soils :- Saline soils defined as soils having a conductivity of the saturation extract greater than 4 dS m-1 and an exchangeable sodium percentage less than 15 Saline soils defined as soils having a conductivity of the saturation extract greater than 4 dS m-1 and an exchangeable sodium percentage less than 15. The pH is usually less than 8.5. -

Managing Soil Salinity Tony Provin and J.L
E-60 3-12 Managing Soil Salinity Tony Provin and J.L. Pitt* f your soil has a high salinity content, the plants ing may cause salts to accumulate in both surface growing there will not be as vigorous as they would and underground waters. The surface runoff of these Ibe in normal soils. Seeds will germinate poorly, dissolved salts is what gives the salt content to our if at all, and the plants will grow slowly or become oceans and lakes. Fertilizers and organic amendments stunted. If the salinity concentration is high enough, also add salts to the soil. the plants will wilt and die, no matter how much you water them. Effects of salts on plants Routine soil testing can identify your soil’s salinity As soils become more saline, plants become unable levels and suggest measures you can take to correct to draw as much water from the soil. This is because the specific salinity problem in your soil. the plant roots contain varying concentrations of ions (salts) that create a natural flow of water from the soil Salinity and salt into the plant roots. The terms salt and salinity are often used inter- As the level of salinity in the soil nears that of the changeably, and sometimes incorrectly. A salt is sim- roots, however, water becomes less and less likely to ply an inorganic mineral that can dissolve in water. enter the root. In fact, when the soil salinity levels are Many people associate salt with sodium chloride— high enough, the water in the roots is pulled back into common table salt. -

Methods of Analysis Soil Sampling
Soil Sampling and Methods of Analysis Second Edition ß 2006 by Taylor & Francis Group, LLC. In physical science the first essential step in the direction of learning any subject is to find principles of numerical reckoning and practicable methods for measuring some quality connected with it. I often say that when you can measure what you are speaking about, and express it in numbers, you know something about it; but when you cannot measure it, when you cannot express it in numbers, your knowledge is of a meagre and unsatisfactory kind; it may be the beginning of knowledge, but you have scarcely in your thoughts advanced to the state of science, whatever the matter may be. Lord Kelvin, Popular Lectures and Addresses (1891–1894), vol. 1, Electrical Units of Measurement Felix qui potuit rerum cognoscere causas. Happy the man who has been able to learn the causes of things. Virgil: Georgics (II, 490) ß 2006 by Taylor & Francis Group, LLC. Soil Sampling and Methods of Analysis Second Edition Edited by M.R. Carter E.G. Gregorich Canadian Society of Soil Science ß 2006 by Taylor & Francis Group, LLC. CRC Press Taylor & Francis Group 6000 Broken Sound Parkway NW, Suite 300 Boca Raton, FL 33487-2742 © 2008 by Taylor & Francis Group, LLC CRC Press is an imprint of Taylor & Francis Group, an Informa business No claim to original U.S. Government works Printed in the United States of America on acid-free paper 10 9 8 7 6 5 4 3 2 1 International Standard Book Number-13: 978-0-8493-3586-0 (Hardcover) This book contains information obtained from authentic and highly regarded sources. -

Saline and Alkali Soils Are Soils That Saline Soil
282 YEARBOOK OF AGRICULTURE 1957 gions of an arid or a scmiarid climate. Under humid conditions, the soluble salts originally present in soil materials and those formed by the weathering of Saline and minerals generally are carried down- ward into the ground water and are transported ultimately by streams to Alkali Soils the oceans. In arid regions, leaching and trans- C. A. Bower and Milton Fireman portation of salts to the oceans is not so complete as in humid regions. Leach- Saline and alkali conditions ing is usually local in nature, and solu- ble salts may not be transported far. lower the productivity and This occurs because there is less rain- value of large areas of agri- fall available to leach and transport the salts and because the high evapo- cultural land in the United ration and plant transpiration rates States—an estimated one- in arid climates tend further to con- centrate the salts in soils and surface fourth of our 29 million acres waters. of irrigated land and less ex- Weathering of primary minerals is the indirect source of nearly all soluble tensive acreages of nonirrigated salts, but there may be a few instances crop and pasture lands. in which enough salts have accumu- lated from this source alone to form a Saline and alkali soils are soils that saline soil. Saline soils usually occur in have been harmed by soluble salts, places that receive salts from other consisting mainly of sodium, calcium, locations; water is the main carrier. magnesium, chloride, and sulfate and secondarily of potassium, bicarbonate, RESTRICTED DRAINAGE usually con- carbonate, nitrate, and boron. -

Chapter 12 Leaching and Rootzone Salinity Control
CHAPTER 12 saimr nil LEACHING AND ROOTZONE SALINITY CONTROL failles E. Ayars, Glenn J. Hoffman, and Dennis L. Corwin ll',sful water management for salinity control depends on ade leJching, which takes place whenever irrigation and rainfall exceed r; ca pacity to store infiltrated wat r within the crop's rootzone. In dregions, rainfall normaLly results in enough leaching to flush salt dl' rootzone. In subhumid and drier regions, irrigation water that the crop's water requirements may need to be applied to ensure 'Jle leaching. Depending on the salinity control needed, leaching ur continuously or at intervals of a few weeks to a few years. (TOP'S water requirement and salinity control must be prime con allon in places where salinity poses a hazard. Proper irrigation the soil's water deficit without a wasteful and potentially harmful l mps need water from irrigation and rainfall to control soil salin mJucing drainage (leaching). As discussed in this chapter, leaching r'move enough salt to prevent it from accumulating in the rootzone th crop's salt-tolerance level. Chemical reactions in the soil affect i!1uun t of salt leached, which may be greater than, equal to, or less t\1t.' amount of salt added by irrigation water. 'amount of irrigation water needed to meet the crop's water require r~'3 n be calcula t d from a wat balance of the crop rootzone. The !low' of water into the crop's rootzone are irrigation, rainfall, and Jrd now from the groundwater. The depths of each are expressed in 371 372 AGRICULTURAL SALINITY ASSESSM E T AND MANAGEMENT equations as OJ, 0" and Og' respectively. -

Soil Salinity: Effect on Vegetable Crop Growth. Management Practices to Prevent and Mitigate Soil Salinization
horticulturae Review Soil Salinity: Effect on Vegetable Crop Growth. Management Practices to Prevent and Mitigate Soil Salinization Rui Manuel Almeida Machado 1,2,* and Ricardo Paulo Serralheiro 1,3 1 ICAAM— Instituto de Ciências Agrárias e Ambientais Mediterrânicas; Universidade de Évora, Pólo da Mitra, Apartado 94, 7006-554 Évora, Portugal 2 Departamento de Fitotecnia, Escola de Ciência Tecnologia, Universidade de Évora, Pólo da Mitra, Apartado 94, 7006-554 Évora, Portugal 3 Departamento de Engenharia Rural, Escola de Ciência Tecnologia, Universidade de Évora, Pólo da Mitra, Apartado 94, 7006-554 Évora, Portugal; [email protected] * Correspondence: [email protected] Academic Editors: Arturo Alvino and Maria Isabel Freire Ribeiro Ferreira Received: 11 January 2017; Accepted: 26 April 2017; Published: 3 May 2017 Abstract: Salinity is a major problem affecting crop production all over the world: 20% of cultivated land in the world, and 33% of irrigated land, are salt-affected and degraded. This process can be accentuated by climate change, excessive use of groundwater (mainly if close to the sea), increasing use of low-quality water in irrigation, and massive introduction of irrigation associated with intensive farming. Excessive soil salinity reduces the productivity of many agricultural crops, including most vegetables, which are particularly sensitive throughout the ontogeny of the plant. The salinity −1 threshold (ECt) of the majority of vegetable crops is low (ranging from 1 to 2.5 dS m in saturated soil extracts) and vegetable salt tolerance decreases when saline water is used for irrigation. The objective of this review is to discuss the effects of salinity on vegetable growth and how management practices (irrigation, drainage, and fertilization) can prevent soil and water salinization and mitigate the adverse effects of salinity. -

Vadose Zone Characterization and Monitoring Current Technologies, Applications, and Future Developments
3 Vadose Zone Characterization and Monitoring Current Technologies, Applications, and Future Developments Boris Faybishenko Contributors: M. Bandurraga, M. Conrad, P. Cook, C. Eddy-Dilek, L. Everett, FRx Inc. of Cincinnati, T. Hazen, S. Hubbard, A.R. Hutter, P. Jordan, C. Keller, F.J. Leij, N. Loaiciga, E.L. Majer, L. Murdoch, S. Renehan, B. Riha, J. Rossabi, Y. Rubin, A. Simmons, S. Weeks, C.V. Williams INTRODUCTION NEEDS FOR VADOSE ZONE CHARACTERIZATION AND MONITORING Vadose zone characterization and monitoring are essential for: • Development of a complete and accurate assessment of the inven- tory, distribution, and movement of contaminants in unsaturated- saturated soils and rocks. • Development of improved predictive methods for liquid flow and contaminant transport. • Design of remediation systems (barrier systems, stabilization of buried wastes in situ, cover systems for waste isolation, in situ treat- ment barriers of dispersed contaminant plumes, bioreactive treat- ment methods of organic solvents in sediments and groundwater). • Design of chemical treatment technologies to destroy or immobilize highly concentrated contaminant sources (metals, radionuclides, explosive residues, and solvents) accumulated in the subsurface. 133 134 VADOSE ZONE SCIENCE AND TECHNOLOGY SOLUTIONS Development of appropriate conceptual models of water flow and chemical transport in the vadose zone soil-rock formation is critical for developing adequate predictive modeling methods and designing cost- effective remediation techniques. These conceptual models of unsatu- rated heterogeneous soils must take into account the processes of preferential and fast water seepage and contaminant transport toward the underlying aquifer. Such processes are enhanced under episodic natural precipitation, snowmelt, and extreme chemistry of waste leaks from tanks, cribs, and other surface sources. -

Measuring Soil Salinity
Measuring soil salinity Viti-note Summary: Salinity is a measure of the concentration Salinity in the root zone often comes of soluble salts in the soil. The most from a saline watertable; therefore the • Method common salt is sodium chloride: however, subsoil, and possibly a deeper level soil • Interpreting results others include bicarbonates, sulphates sample, should also be measured. and carbonates of calcium, potassium • Useful conversions and magnesium. Some salts are useful, Method e.g. many fertilizers are in a salt form, but too much salt of any kind is detrimental 1. Take three surface soil and three to plants and other organisms. subsoil samples from each site (as described in points 1-5 the Taking soil Vitis vinifera varieties are moderately samples activity guide in this Vitinote tolerant of salinity (i.e. high total salts). series). Make sure surface soil and However, a concentration of salts in the subsoil are not combined so that they root zone that is too high can damage can be analysed separately. plant health and reduce crop yields. A very high concentration of soluble salts 2. Crush large aggregates and remove can kill vines. Measurement of soil salinity any gravel so that you have a fine mix is generally used to determine the salt to test. status of a soil, particularly if vines are 3. Make sure to refer to instrument showing salt toxicity symptoms. It is instructions and periodically calibrate also used to gauge the impact on soils your EC meter. irrigated with saline water, particularly in 4. Unscrew jar lid and fill the lid level combination with deficit irrigation. -

An APLD Guide to Sustainable Soils
An APLD Guide to Sustainable Soils We Define Landscape Design www.apld.org 1 APLD is committed to the principles of sustainability as the foundation for our collective landscape design practice. Healthy, appropriate soils, sustainably generated and maintained, are key to a strong, diverse ecosystem and are essential for a successful planting design. The Properties of Soil Soil has physical, chemical and biological properties, all combining to provide exquisite complexity for supporting plant life. Physical Properties of Soil The physical components of soil include liquids, The mineral solids are sand, silt, and clay, usually gases, and mineral and organic solids. mixed in varying concentrations that define the texture of the soil. The largest particles are sand, followed by silt and clay, in that order. The comparative sizes of the particles are shown below. U.S. Dept. of Agriculture National Resources Conservation Service U.S. Dept. of Agriculture National Resources Conservation Service 2 There are twelve main classes of soil texture, ranging The organic solids are carbon-based and derive from from blends of the mineral solids, with a combination the decaying remains of plant and animal life in the of approximately equal parts silt and sand, no more soil. They contribute to the structure of the soil by than 20% clay, and some organic solids being among working with clay to encourage the development the best types of soils. The diagram below illustrates of soil aggregates and cement the particles, thus the 12 main soil textures. stabilizing soil structure. Organic solids also improve the ability of the soil to hold water.