Community Composition of Elasmobranch Fishes Utilizing Intertidal Sand Flats in Moreton Bay, Queensland, Australia1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Species Bathytoshia Brevicaudata (Hutton, 1875)
FAMILY Dasyatidae Jordan & Gilbert, 1879 - stingrays SUBFAMILY Dasyatinae Jordan & Gilbert, 1879 - stingrays [=Trygonini, Dasybatidae, Dasybatidae G, Brachiopteridae] GENUS Bathytoshia Whitley, 1933 - stingrays Species Bathytoshia brevicaudata (Hutton, 1875) - shorttail stingray, smooth stingray Species Bathytoshia centroura (Mitchill, 1815) - roughtail stingray Species Bathytoshia lata (Garman, 1880) - brown stingray Species Bathytoshia multispinosa (Tokarev, in Linbergh & Legheza, 1959) - Japanese bathytoshia ray GENUS Dasyatis Rafinesque, 1810 - stingrays Species Dasyatis chrysonota (Smith, 1828) - blue stingray Species Dasyatis hastata (DeKay, 1842) - roughtail stingray Species Dasyatis hypostigma Santos & Carvalho, 2004 - groovebelly stingray Species Dasyatis marmorata (Steindachner, 1892) - marbled stingray Species Dasyatis pastinaca (Linnaeus, 1758) - common stingray Species Dasyatis tortonesei Capapé, 1975 - Tortonese's stingray GENUS Hemitrygon Muller & Henle, 1838 - stingrays Species Hemitrygon akajei (Muller & Henle, 1841) - red stingray Species Hemitrygon bennettii (Muller & Henle, 1841) - Bennett's stingray Species Hemitrygon fluviorum (Ogilby, 1908) - estuary stingray Species Hemitrygon izuensis (Nishida & Nakaya, 1988) - Izu stingray Species Hemitrygon laevigata (Chu, 1960) - Yantai stingray Species Hemitrygon laosensis (Roberts & Karnasuta, 1987) - Mekong freshwater stingray Species Hemitrygon longicauda (Last & White, 2013) - Merauke stingray Species Hemitrygon navarrae (Steindachner, 1892) - blackish stingray Species -

Extinction Risk and Conservation of the World's Sharks and Rays
RESEARCH ARTICLE elife.elifesciences.org Extinction risk and conservation of the world’s sharks and rays Nicholas K Dulvy1,2*, Sarah L Fowler3, John A Musick4, Rachel D Cavanagh5, Peter M Kyne6, Lucy R Harrison1,2, John K Carlson7, Lindsay NK Davidson1,2, Sonja V Fordham8, Malcolm P Francis9, Caroline M Pollock10, Colin A Simpfendorfer11,12, George H Burgess13, Kent E Carpenter14,15, Leonard JV Compagno16, David A Ebert17, Claudine Gibson3, Michelle R Heupel18, Suzanne R Livingstone19, Jonnell C Sanciangco14,15, John D Stevens20, Sarah Valenti3, William T White20 1IUCN Species Survival Commission Shark Specialist Group, Department of Biological Sciences, Simon Fraser University, Burnaby, Canada; 2Earth to Ocean Research Group, Department of Biological Sciences, Simon Fraser University, Burnaby, Canada; 3IUCN Species Survival Commission Shark Specialist Group, NatureBureau International, Newbury, United Kingdom; 4Virginia Institute of Marine Science, College of William and Mary, Gloucester Point, United States; 5British Antarctic Survey, Natural Environment Research Council, Cambridge, United Kingdom; 6Research Institute for the Environment and Livelihoods, Charles Darwin University, Darwin, Australia; 7Southeast Fisheries Science Center, NOAA/National Marine Fisheries Service, Panama City, United States; 8Shark Advocates International, The Ocean Foundation, Washington, DC, United States; 9National Institute of Water and Atmospheric Research, Wellington, New Zealand; 10Global Species Programme, International Union for the Conservation -

Malaysia National Plan of Action for the Conservation and Management of Shark (Plan2)
MALAYSIA NATIONAL PLAN OF ACTION FOR THE CONSERVATION AND MANAGEMENT OF SHARK (PLAN2) DEPARTMENT OF FISHERIES MINISTRY OF AGRICULTURE AND AGRO-BASED INDUSTRY MALAYSIA 2014 First Printing, 2014 Copyright Department of Fisheries Malaysia, 2014 All Rights Reserved. No part of this publication may be reproduced or transmitted in any form or by any means, electronic, mechanical, including photocopy, recording, or any information storage and retrieval system, without prior permission in writing from the Department of Fisheries Malaysia. Published in Malaysia by Department of Fisheries Malaysia Ministry of Agriculture and Agro-based Industry Malaysia, Level 1-6, Wisma Tani Lot 4G2, Precinct 4, 62628 Putrajaya Malaysia Telephone No. : 603 88704000 Fax No. : 603 88891233 E-mail : [email protected] Website : http://dof.gov.my Perpustakaan Negara Malaysia Cataloguing-in-Publication Data ISBN 978-983-9819-99-1 This publication should be cited as follows: Department of Fisheries Malaysia, 2014. Malaysia National Plan of Action for the Conservation and Management of Shark (Plan 2), Ministry of Agriculture and Agro- based Industry Malaysia, Putrajaya, Malaysia. 50pp SUMMARY Malaysia has been very supportive of the International Plan of Action for Sharks (IPOA-SHARKS) developed by FAO that is to be implemented voluntarily by countries concerned. This led to the development of Malaysia’s own National Plan of Action for the Conservation and Management of Shark or NPOA-Shark (Plan 1) in 2006. The successful development of Malaysia’s second National Plan of Action for the Conservation and Management of Shark (Plan 2) is a manifestation of her renewed commitment to the continuous improvement of shark conservation and management measures in Malaysia. -

Age and Growth of the Endemic Xingu River Stingray Potamotrygon Leopoldi Validated Using Fluorescent Dyes
Journal of Fish Biology (2018) 92, 1985–1999 doi:10.1111/jfb.13635, available online at wileyonlinelibrary.com Age and growth of the endemic Xingu River stingray Potamotrygon leopoldi validated using fluorescent dyes P. Charvet*, F. M. Santana†,K.L.DeLima‡ and R. Lessa‡§ *Departamento de Ecologia e Sistemática (CCEN), Universidade Federal da Paraíba (UFPB), Cidade Universitária, João Pessoa, PB, CEP 58051-090, Brazil, †Unidade Acadêmica de Serra Talhada (UAST), Universidade Federal Rural de Pernambuco (UFRPE), Serra Talhada, PE, CEP 56903-970, Brazil and ‡Departamento de Pesca e Aqüicultura (DEPAq), Universidade Federal Rural de Pernambuco (UFRPE), Dois Irmãos, Recife, PE, CEP 52171-900, Brazil (Received 22 December 2017, Accepted 5 April 2018) Between 2003 and 2005, vertebrae of 151 Xingu River Potamotrygon leopoldi (Potamotrygonidae) (75 males and 76 females) were analysed to derive a growth curve for this species. The disc width (WD) was significantly different between sexes, with females measuring 149–700 mm WD and males 109–500 mm WD. The average percentage error for vertebrae readings of the whole sample was 2·7%. The marginal increment ratio (RMI) showed an increasing trend with the highest value in November, decreasing from December on. The majority of vertebrae displaying RMI zero, occurred in September, but the annual periodicity of ring deposition throughout the year was not conclusive. Tetracycline (TCN) injected specimens were held in captivity for 13 months and displayed a fluorescent mark in vertebrae confirming a yearly periodicity of band pair formation with the translucent ring deposited in September–October. The Akaike information criterion (AIC) showed that, among the seven models considered, the best fit was obtained for the von Bertalanffy modified with W0 (where W0 = WD at birth) for both sexes. -

Extinction Risk and Conservation of the World's Sharks and Rays
Extinction risk and conservation of the world's sharks and rays Nicholas K. Dulvy1*, Sarah L. Fowler2, John A. Musick3, Rachel D. Cavanagh4, Peter M. Kyne5, Lucy R. Harrison1, John K. Carlson6, Lindsay N. K. Davidson1, Sonja V. Fordham7, Malcolm P. Francis8, Caroline M. Pollock9, Colin A. Simpfendorfer10, George H. Burgess11, Kent E. Carpenter12, Leonard J. V. Compagno13, David A. Ebert14, Claudine Gibson2, Michelle R. Heupel15, Suzanne R. Livingstone16, Jonnell C. Sanciangco12, John D. Stevens17, Sarah Valenti2, & William T. White17 1IUCN Species Survival Commission Shark Specialist Group and Earth to Ocean Research Group, Department of Biological Sciences, Simon Fraser University, Burnaby, British Colombia V5A 1S6, Canada; 2IUCN Species Survival Commission Shark Specialist Group, NatureBureau International, 36 Kingfisher Court, Hambridge Road, Newbury RG14 5SJ, UK; 3Virginia Institute of Marine Science, Greate Road, Gloucester Point, VA 23062, USA; 4British Antarctic Survey, Natural Environment Research Council, Madingley Road, Cambridge CB3 0ET, UK; 5Research Institute for the Environment and Livelihoods, Charles Darwin University, Darwin, Northern Territory 0909, Australia; 6NOAA/National Marine Fisheries Service, Southeast Fisheries Science Center, 3500 Delwood Beach Road, Panama City, FL 32408, USA; 7Shark Advocates International, The Ocean Foundation, 1990 M Street, NW, Suite 250, Washington, DC 20036, USA; 8National Institute of Water and Atmospheric Research, Private Bag 14901, Wellington, New Zealand; 9Species Programme, IUCN, -

Elasmobranch Biodiversity, Conservation and Management Proceedings of the International Seminar and Workshop, Sabah, Malaysia, July 1997
The IUCN Species Survival Commission Elasmobranch Biodiversity, Conservation and Management Proceedings of the International Seminar and Workshop, Sabah, Malaysia, July 1997 Edited by Sarah L. Fowler, Tim M. Reed and Frances A. Dipper Occasional Paper of the IUCN Species Survival Commission No. 25 IUCN The World Conservation Union Donors to the SSC Conservation Communications Programme and Elasmobranch Biodiversity, Conservation and Management: Proceedings of the International Seminar and Workshop, Sabah, Malaysia, July 1997 The IUCN/Species Survival Commission is committed to communicate important species conservation information to natural resource managers, decision-makers and others whose actions affect the conservation of biodiversity. The SSC's Action Plans, Occasional Papers, newsletter Species and other publications are supported by a wide variety of generous donors including: The Sultanate of Oman established the Peter Scott IUCN/SSC Action Plan Fund in 1990. The Fund supports Action Plan development and implementation. To date, more than 80 grants have been made from the Fund to SSC Specialist Groups. The SSC is grateful to the Sultanate of Oman for its confidence in and support for species conservation worldwide. The Council of Agriculture (COA), Taiwan has awarded major grants to the SSC's Wildlife Trade Programme and Conservation Communications Programme. This support has enabled SSC to continue its valuable technical advisory service to the Parties to CITES as well as to the larger global conservation community. Among other responsibilities, the COA is in charge of matters concerning the designation and management of nature reserves, conservation of wildlife and their habitats, conservation of natural landscapes, coordination of law enforcement efforts as well as promotion of conservation education, research and international cooperation. -

A Review of Longnose Skates Zearaja Chilensisand Dipturus Trachyderma (Rajiformes: Rajidae)
Univ. Sci. 2015, Vol. 20 (3): 321-359 doi: 10.11144/Javeriana.SC20-3.arol Freely available on line REVIEW ARTICLE A review of longnose skates Zearaja chilensis and Dipturus trachyderma (Rajiformes: Rajidae) Carolina Vargas-Caro1 , Carlos Bustamante1, Julio Lamilla2 , Michael B. Bennett1 Abstract Longnose skates may have a high intrinsic vulnerability among fishes due to their large body size, slow growth rates and relatively low fecundity, and their exploitation as fisheries target-species places their populations under considerable pressure. These skates are found circumglobally in subtropical and temperate coastal waters. Although longnose skates have been recorded for over 150 years in South America, the ability to assess the status of these species is still compromised by critical knowledge gaps. Based on a review of 185 publications, a comparative synthesis of the biology and ecology was conducted on two commercially important elasmobranchs in South American waters, the yellownose skate Zearaja chilensis and the roughskin skate Dipturus trachyderma; in order to examine and compare their taxonomy, distribution, fisheries, feeding habitats, reproduction, growth and longevity. There has been a marked increase in the number of published studies for both species since 2000, and especially after 2005, although some research topics remain poorly understood. Considering the external morphological similarities of longnose skates, especially when juvenile, and the potential niche overlap in both, depth and latitude it is recommended that reproductive seasonality, connectivity and population structure be assessed to ensure their long-term sustainability. Keywords: conservation biology; fishery; roughskin skate; South America; yellownose skate Introduction Edited by Juan Carlos Salcedo-Reyes & Andrés Felipe Navia Global threats to sharks, skates and rays have been 1. -

The Ecology and Biology of Stingrays (Dasyatidae)� at Ningaloo Reef, Western � Australia
The Ecology and Biology of Stingrays (Dasyatidae) at Ningaloo Reef, Western Australia This thesis is presented for the degree of Doctor of Philosophy of Murdoch University 2012 Submitted by Owen R. O’Shea BSc (Hons I) School of Biological Sciences and Biotechnology Murdoch University, Western Australia Sponsored and funded by the Australian Institute of Marine Science Declaration I declare that this thesis is my own account of my research and contains as its main content, work that has not previously been submitted for a degree at any tertiary education institution. ........................................ ……………….. Owen R. O’Shea Date I Publications Arising from this Research O’Shea, O.R. (2010) New locality record for the parasitic leech Pterobdella amara, and two new host stingrays at Ningaloo Reef, Western Australia. Marine Biodiversity Records 3 e113 O’Shea, O.R., Thums, M., van Keulen, M. and Meekan, M. (2012) Bioturbation by stingray at Ningaloo Reef, Western Australia. Marine and Freshwater Research 63:(3), 189-197 O’Shea, O.R, Thums, M., van Keulen, M., Kempster, R. and Meekan, MG. (Accepted). Dietary niche overlap of five sympatric stingrays (Dasyatidae) at Ningaloo Reef, Western Australia. Journal of Fish Biology O’Shea, O.R., Meekan, M. and van Keulen, M. (Accepted). Lethal sampling of stingrays (Dasyatidae) for research. Proceedings of the Australian and New Zealand Council for the Care of Animals in Research and Teaching. Annual Conference on Thinking outside the Cage: A Different Point of View. Perth, Western Australia, th th 24 – 26 July, 2012 O’Shea, O.R., Braccini, M., McAuley, R., Speed, C. and Meekan, M. -

Redalyc.A Review of Longnose Skates Zearaja Chilensis and Dipturus Trachyderma (Rajiformes: Rajidae)
Universitas Scientiarum ISSN: 0122-7483 [email protected] Pontificia Universidad Javeriana Colombia Vargas-Caro, Carolina; Bustamante, Carlos; Lamilla, Julio; Bennett, Michael B. A review of longnose skates Zearaja chilensis and Dipturus trachyderma (Rajiformes: Rajidae) Universitas Scientiarum, vol. 20, núm. 3, 2015, pp. 321-359 Pontificia Universidad Javeriana Bogotá, Colombia Available in: http://www.redalyc.org/articulo.oa?id=49941379004 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Univ. Sci. 2015, Vol. 20 (3): 321-359 doi: 10.11144/Javeriana.SC20-3.arol Freely available on line REVIEW ARTICLE A review of longnose skates Zearaja chilensis and Dipturus trachyderma (Rajiformes: Rajidae) Carolina Vargas-Caro1 , Carlos Bustamante1, Julio Lamilla2 , Michael B. Bennett1 Abstract Longnose skates may have a high intrinsic vulnerability among fishes due to their large body size, slow growth rates and relatively low fecundity, and their exploitation as fisheries target-species places their populations under considerable pressure. These skates are found circumglobally in subtropical and temperate coastal waters. Although longnose skates have been recorded for over 150 years in South America, the ability to assess the status of these species is still compromised by critical knowledge gaps. Based on a review of 185 publications, a comparative synthesis of the biology and ecology was conducted on two commercially important elasmobranchs in South American waters, the yellownose skate Zearaja chilensis and the roughskin skate Dipturus trachyderma; in order to examine and compare their taxonomy, distribution, fisheries, feeding habitats, reproduction, growth and longevity. -

WCPTOC3.CHP:Corel VENTURA
click for previous page INDEX OF SCIENTIFIC AND VERNACULAR NAMES Explanation of the System Italics : Valid scientific names (genera and species). Italics : Synonyms (genera and species), misidentifications. ROMAN : Family names. ROMAN : Names of divisions, classes, subclasses, orders, suborders, and subfamilies. Roman: FAO and local names. 2040 The Living Marine Resources of the Western Central Pacific A Alepocephalids ...................1895 Alepocephalus agassizii ............1888 abbotti, Notacanthus .............. 1628 Alepocephalus australis ............1888 abbreviata, Harengula .............1796 Alepocephalus bairdii ............. 1888 abbreviatus, Gonorynchus ...........1826 Alepocephalus longiceps ............1888 abei, Chaunax .................. 2020 Alfonsinos ......................1578 abnormis, Ilisha ................. 1758 See also Vol. 4 ACANTHURIDAE ..............1610, 1967 Alicefranche....................1712 See also Vol. 6 Alice taches d’or ..................1709 ACANTHUROIDEI.................1609 Allenbatrachus grunniens ...........2001 See also Vol. 6 Allenbatrachus reticulatus...........2001 Acetes .......................1753 Alosa alburnus .................. 1816 ACROPOMATIDAE ................1584 Alosa brevis ................... 1802 See also Vol. 4 Alosachata.....................1791 acuta, Dussumieria............ 1792-1793 Alosa malayana ................. 1802 acutus, Arius ...................1839 Aloseàgrosyeux..................1763 ADRIANICHTHYIDAE ...............1573 Aloseàmuseaucourt................1791 See -
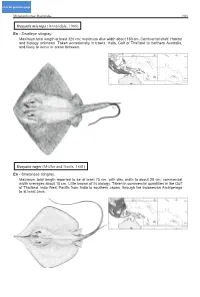
WCPTOC3.CHP:Corel VENTURA
click for previous page Myliobatiformes: Dasyatidae 1501 Dasyatis microps (Annandale, 1908) En - Smalleye stingray. Maximum total length at least 320 cm; maximum disc width about 180 cm. Continental shelf. Habitat and biology unknown. Taken occasionally in trawls. India, Gulf of Thailand to northern Australia, and likely to occur in areas between. Dasyatis zugei (Müller and Henle, 1841) En - Sharpnose stingray. Maximum total length reported to be at least 75 cm, with disc width to about 29 cm; commercial width averages about 18 cm. Little known of its biology. Taken in commercial quantities in the Gulf of Thailand. Indo-West Pacific from India to southern Japan, through the Indonesian Archipelago to at least Java. 1502 Batoid Fishes Himantura imbricata (Bloch and Schneider, 1801) En - Scaly whipray. Maximum total length about 65 cm; maximum disc width 22 cm. Distribution through Indo-Malay Archipelago not well defined (regularly confused with Himantura walga). Thought to occur from the Red Sea to Java. Himantura marginata (Blyth, 1860) En - Blackedge whipray. Maximum total length at least 345 cm; maximum disc width 179 cm. Poorly known species. Thought to occur off India, Sri Lanka, and Myanmar, may venture into Indonesian waters; possibly off Mozambique. Myliobatiformes: Dasyatidae 1503 Himantura oxyrhynchus (Sauvage, 1878) En - Marbled whipray. Maximum total length at least 126 cm; maximum disc width 36 cm. A fresh-water species; can also be found in estuaries. Unknown commercial use. Cambodia, Thailand, and Borneo. Himantura signifer Compagno and Roberts, 1982 En - Pale whipray. Maximum total length at least 178 cm; maximum disc width 38 cm. A fresh-water species; can also be found in estuaries. -

Cape York Peninsula Natural Resource Management Region
Threats to priority species of the Cape York Peninsula region. On day one of the regional workshop, participants used their knowledge of individual species to identify the major and minor regional threats that are affecting each species (Table 2). Table 2a. Threats to Cape York Peninsula NRM region priority plant species. a) Threat name (specific enough to act on), b) importance of the threat to the species: M = major importance, m = minor importance; c) how the threat impacts on the species; and d) threat details. RP = Recovery plan, AP = Action plan, SMP = Species management profile, TWN = technical workshop note. Species name Common a) Threat name b) Priority c) Threat impacts d) Threat details name Aponogeton Ferals - Pigs M Loss and / or removal of The movement and rooting of feral pigs removes individuals of this species and is a cuneatus individuals major threat. Weeds M Competition Riparian and aquatic weeds, such as Hymenachne , can dominate the habitat and out- compete A. cuneatus . Linear infrastructure m Loss and / or removal of The development of creek crossings can remove individuals of this species. development individuals Collectors m Loss and / or removal of The removal of individuals by collectors is a minor threat to the species. individuals Aponogeton wire grass Weeds M Competition Riparian and aquatic weeds, such as Hymenachne , can dominate the habitat and out- queenslandicus compete A. queenslandicus . Ferals - Pigs M Loss and / or removal of The movement and rooting of feral pigs removes individuals of this species and is a individuals major threat. Clearing of vegetation m Loss of habitat The clearing of vegetation around freshwater bodies, where A.